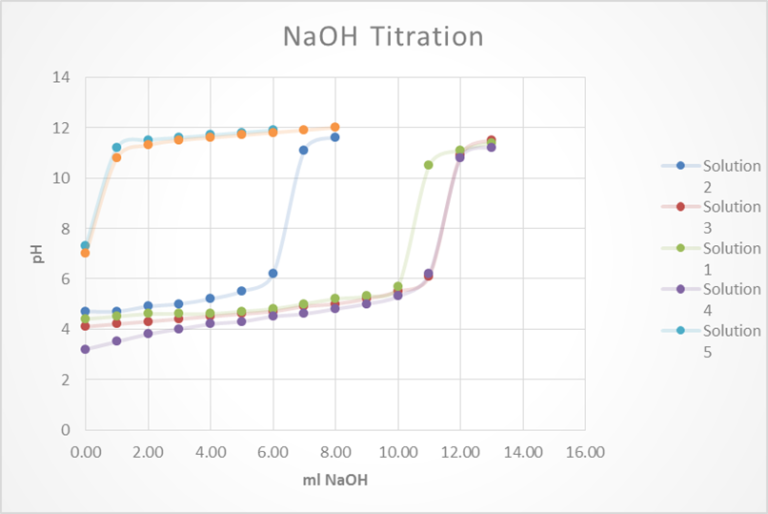
Polarity
For the first part of the experiment, predictions where made on how different solutes would dissolve in different solvents. The two solvents used were water which is a polar and hexane which is a non polar. Knowing that polar molecules dissolve polar molecules or molecules with a charge, but they do not interact with none polar. The same is true for none polar molecules dissolving none polar molecules. After reviewing each molecular structure of each solute prediction were made based on based on the polarity of the molecule and the solvent. Please see table 1: Solute + Solvent, for predictions and actual results.

For the next part Iodine was added to a mixture of water and hexane. My prediction was that the Iodine would mix with the hexane because both molecules are non-polar, and there would be very little interaction with the water and the iodine due to the fact that water is a polar molecule. After conducting this test my observations was that there were 3 visible layers the top layer was dark purple (a mixture of Iodine + Hexane). Because the hexane iodine mixture is less dense then water it would rise to the top of the test tube. In the middle there was a clear light gold aqueous layer made up of mostly water. I believe a small amount of iodine dissolved in the water, which would explain the color change. All the way at the bottom was where the iodine that was left over, and had precipitated to the bottom. After removing the top hexane mixture, I added pure hexane to the aqueous solution and I noticed that the dark purple layer increased, this was most likely due to the chemical reaction with Iodine that had dissolved in water. But the aqueous clear gold layer did not mix with the new top dark purple layer.
The next experiment conducted was Copper (II) Sulfate was added to a mixture of water and hexane. There were 3 visible layers after mixing the solution. The Copper (II) Sulfate was on the bottom, water was in the middle, and hexane was on top. The Copper (II) Sulfate was blue and both water and hexane were clear. Copper (II) Sulfate is not soluble in hexane because there is nothing attracting these two molecules together. Where as the Copper (II) Sulfate can dissolve in water because water is a polar molecule and similar to sodium chloride it is able to break apart the Copper (II) Sulfate into is positive and negatively charged ions.
For the final part of this experiment sodium chloride to a mixture of water and hexane, because the sodium chloride has a ionic bond then the neutrally charged hexane would not be able to dissolve this salt and the salt will fall right though it. Once the salts reaches the water at the bottom of the test tube it will start to be broken down into its ions, and as a result start to dissolve in the water. The water will break up the salt until it is fully saturated the remainder of the salt will precipitate to the bottom of the test tube. After the aqueous water and salt mixture is removed from the test tube, the water was evaporated. With the water gone the only thing remaining was the salt crystals.