Energy vs Power
What’s the difference between energy vs power? In the realm of physics and engineering, the concepts of energy and power are fundamental but often misunderstood. While they are closely related, they represent distinct aspects of physical systems. Energy is the ability to do work, while power is the rate at which work is done or energy is transferred. To grasp these concepts fully, it is essential to delve into the various forms of energy, measures, and efficiency considerations.
What is Energy?
Energy, in its simplest form, is the capacity to do work while providing a usable level of power. Work, in a physical sense, is the transfer of energy from one object to another, typically resulting in some form of motion or change. Energy comes in several forms, each with its unique characteristics and properties.
What is the conservation of energy?
The law of conservation of energy states that energy not be created or destroyed. The most comprehensive form of the conservation-of-energy principle is articulated as the first law of thermodynamics.
Forms of Energy Overview
Energy exists in many different forms. The five forms of energy are as follows:
- Mechanical Energy
- Kinetic Energy
- Potential Energy
- Internal Energy
- Latent (links between molecules)
- Chemical (links between atoms in a molecule)
- Nuclear (links between particles in an atom’s nucleus)
- Electrical Energy
- Electromagnetic Energy
- Electrochemical Energy
What is a simple Definition of Mechanical Energy?
In the realm of physical sciences, mechanical energy comprises both potential and kinetic energy. Mechanical energy is the energy possessed by an object due to its motion (kinetic energy) or its position relative to other objects (potential energy). Mechanical energy plays a pivotal role in renewable energy production. Numerous renewable energy sources depend on mechanical energy for efficient power generation and energy conversion.
Emechanical = Ekinetic+ Epotential
Kinetic Energy:
Kinetic energy is the energy associated with motion, evident in the movement of objects or subatomic particles. All moving objects and particles possess kinetic energy. Examples include a person walking because it’s in motion relative to the ground. Other common examples are a baseball flying through the air, a crumb falling from a table, a moving car, and a charged particle moving in an electric field.

Potential Energy:
The energy an object possesses due to its position relative to other objects, internal stresses, electric charge, and other influencing factors. Consider a book sitting on a table. The book is said to have “potential energy” because if it is nudged off, gravity will accelerate the book, giving the book kinetic energy.

What is Internal Energy in simple words?
The internal energy (U) of a system or object with clearly defined boundaries consists of the combined kinetic energy from molecular motion, potential energy from molecular vibration and electric interactions within molecules, and the energy stored in chemical bonds. It includes thermal energy, which is the energy associated with the random motion of particles in a substance, and chemical energy, which is stored in the bonds between atoms and molecules.
Thermal Energy:
Thermal energy is the energy stored within a system that determines its temperature, while heat is the transfer of this thermal energy. Consider a hot cup of coffee. The coffee is said to possess “thermal energy”, or “heat energy” which is the collective, microscopic, kinetic, and potential energy of the molecules in the coffee.

What does temperature measure?
Temperature is a measure of how much thermal energy something has. The higher the
temperature, the faster the molecules are moving around and/or vibrating, i.e. the more kinetic and
potential energy the molecules have.

Chemical Energy:
Chemical energy is the energy stored in chemical substances, which is released during a chemical reaction as the substances transform into different substances. Examples of chemical energy storage media include batteries, food, and gasoline. Consider the ability of your body to do work. The glucose (blood sugar) in your body is said to have “chemical energy” because the glucose releases energy when chemically reacted (combusted) with oxygen. Similarly, power plants convert the chemical energy in coal into thermal and electrical energy.

Chemical energy is a form of microscopic potential energy, which exists because of the electric and magnetic forces of attraction exerted between the different parts of each molecule. These parts get rearranged in chemical reactions, releasing or adding to this potential energy.
Electrical Energy:
Electrical energy is the form of energy associated with the interactions and movements of electrically charged particles (electric charge), typically electrons in wires, but not limited to them.

Electrical energy is commonly used to power electrical devices and is a crucial form of energy in modern society. The faster the charges move, the more electrical energy they carry. As the electrons move through a “resistor” in the circuit, they interact with the atoms in the resistor very strongly, causing the resistor to heat up – hence delivering energy in the form of heat.
Electrochemical Energy:
Electrochemical energy is the energy stored in chemical bonds that can be released during a chemical reaction. This form of energy is commonly found in batteries, where it is converted into electrical energy. The battery also chemically stores energy, but electricity is also involved, so we say that the battery stores energy “electro-chemically”. Electrochemical energy often involves reactions that transfer electrons, known as redox reactions (reduction-oxidation).

Electromagnetic Energy:
Electromagnetic energy is a form of energy that travels through space as waves. It is responsible for the sensation of sight and is used in various technologies, such as solar panels and lasers. Electromagnetic energy travels in waves across a wide spectrum, ranging from long radio waves to short gamma rays. Devices such as radios and X-ray machines can detect different parts of the electromagnetic spectrum.

The human eye can perceive only a small portion of this electromagnetic spectrum, known as visible light. Light, which is also called “electromagnetic radiation”. Why the fancy term? Because light really can be thought of as oscillating, coupled electric and magnetic fields that travel freely through space. Light may also be thought of as little packets of energy called photons (that is, as particles, instead of waves). The word “photon” derives from the word “photo”, which means “light”.
Sound Energy:
Sound energy is the energy produced by vibrations that travel through a medium, such as air or water. It is the energy responsible for the sensation of hearing and is used in various applications, such as sonar and musical instruments.

Sound waves are compression waves associated with the potential and kinetic energy of air molecules. When an object moves quickly, for example, the head of the drum, it compresses the air nearby, giving that air potential energy. That air then expands, transforming the potential energy into kinetic energy (moving air). The moving air then pushes on and compresses other air, and so on down the chain.
Nuclear Energy:
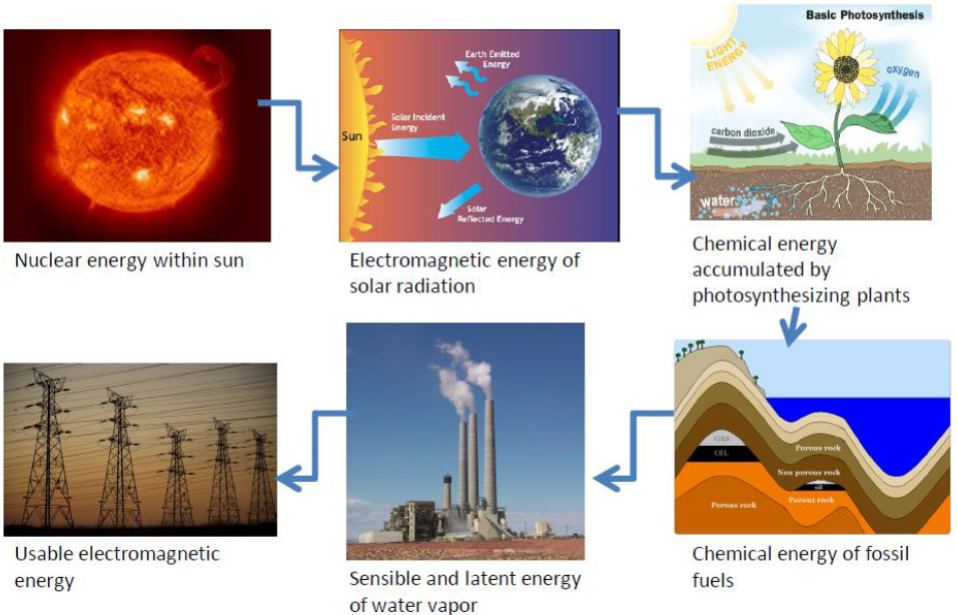
From Einstein’s Theory of Special Relativity (E=MC2). The energy intrinsically stored in a piece of matter at rest equals its mass times the speed of light squared. Nuclear energy is the energy stored in the nucleus of an atom. It is released during nuclear reactions, such as nuclear fission (splitting of atoms) or fusion (merging of atoms), and is used in nuclear power plants to generate electricity.

In the Sun, hydrogen nuclei fuse (combine) together to make helium nuclei, in a process called fusion, which releases energy. In a nuclear reactor, or the interior of the Earth, Uranium nuclei (and certain other heavy elements in the Earth’s interior) split apart, in a process called fission.
Units of Energy
Named after James Prescott Joule, energy is measured in joules (J), which is the standard unit of energy in the International System of Units (SI). One joule is equal to the energy transferred when a force of one newton acts over a distance of one meter. Some popular units for comparing energy include British thermal units (Btu), the erg in the CGS units, and a kilowatt-hour ( kWh).
A kilowatt-hour is a unit of energy, not part of the SI system, equivalent to 3.6 megajoules (MJ) in SI units. It represents the energy delivered by a one-kilowatt power source over one hour. Kilowatt-hours are frequently used as a billing unit for electrical energy provided by electric utilities. See various types of energy measurements below:
- Joule: 1 J=1 N・m=1 kg・m2・s-2=1 V・C
- kilojoule: 1 kJ= 1000 J
- calorie: 1 cal = 4.184 J
- erg: 1 erg = 10-7 J
- kilowatt-hour: 1 kWh=3,600 kJ
- British Thermal Unit: 1 BTU = 1.055056 kJ
- quad of energy: 1 quad = 1015 BTU = 1.055 × 1018 J
What is Energy Transfer in Physics?
Energy can be transferred from one object to another or converted from one form to another. Energy transfer can happen via work done by one system on another or by direct transfer by heat or radiation. For example, when a ball is thrown into the air, the kinetic energy of the thrower’s arm is transferred to the ball, causing it to move upwards. Similarly, when fuel is burned in an engine, the chemical energy stored in the fuel is converted into thermal energy, which is then converted into mechanical energy to move the vehicle.
What is Power?
In physics, power is the rate at which work is done or energy is transferred. It is a measure of how quickly energy is transformed or transferred from one form to another. The unit of power is the watt (W), which is equal to one joule per second.
Power = Work/ Δ Time
Units of Power
The SI unit of power is the watt, symbolized as (W), according to common standards. One watt is equivalent to one joule per second. Remarkably, the unit was named in honor of James Watt, credited with inventing the steam engine’s condenser. Watt also introduced the term “horsepower,” an older unit of power. See various types of power measurements below:
- watt: 1 W = 1 Joule per second
- kilowatt: 1 kW = 103 W
- Megawatt: 1 MW = 106 W
- giga, tera, peta, nano pico-watt= 109,1012,1015,10-9,10-12 W
- horsepower: 1 hp = 745.7 W
- British Thermal Unit per hour: 1 Btu/h = 3.798 MW
Example of Power Usage
- Lifting a mosquito at 1 cm/s : 10-7 W
- Pumping human heart: 1.5 W
- Incandescent light bulb: 60 W
- Human hard physical work: 100 W
- Draft horse hard physical work: 1 kW
- Compact car: 100 kW
- Cruising Boeing 747: 250 MW
- A large coal power plant: 1 GW
- Space Shuttle at take-off: 14 GW
- Humankind in 2005: 11 TW
Efficiency of Devices for Energy Generation and Transfer
Efficiency is a measure of how well a device converts energy from one form to another. It is calculated as the ratio of useful output energy to input energy, expressed as a percentage ( η ≡ Efficiency = Usable Energy Output /Total Energy Input < 1):
Efficiency (%)=(Input Energy/Useful Output Energy)×100%
For example, an incandescent light bulb has an efficiency of around 10%, meaning that only 10% of the electrical energy it consumes is converted into light, while the rest is wasted primarily in heat generated by the resistance.
Example of efficiency – Automotive Engines:
Modern gasoline engines can achieve thermal efficiencies exceeding 50%, yet most road-legal cars typically operate at efficiencies ranging from 20% to 40%. While many engines could potentially achieve higher efficiency, doing so often leads to increased wear and emissions.
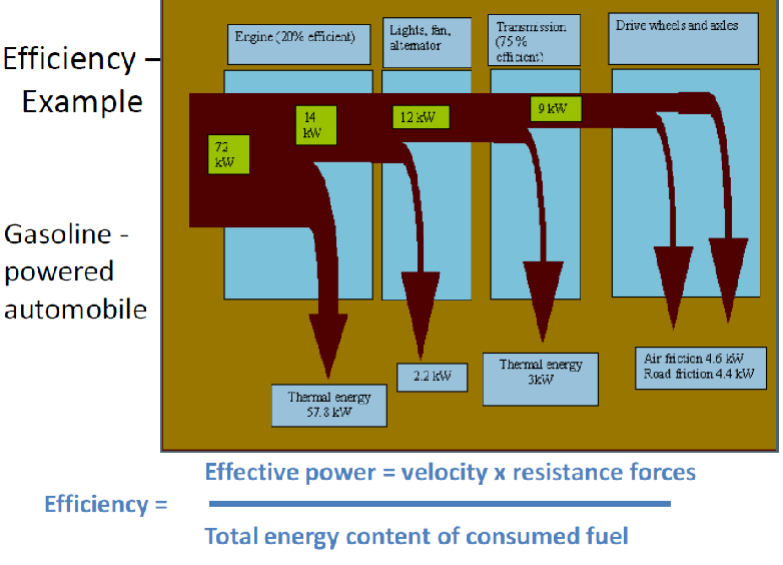
Efficiency of Energy Conversion
Energy conversion efficiency is one of the most important characteristics of any energy system. Efficiency of energy conversion is defined as the ratio of useful energy output (benefit) to energy input (cost). Energy can be classified in terms of quantity and quality. Efficiency is crucial in energy conversion processes, such as power plants or engines. In these systems, not all of the input energy can be converted into useful work, and some is always lost as waste heat and sound. Improving the efficiency of these systems is essential for reducing energy waste and reducing the overall environmental impact.
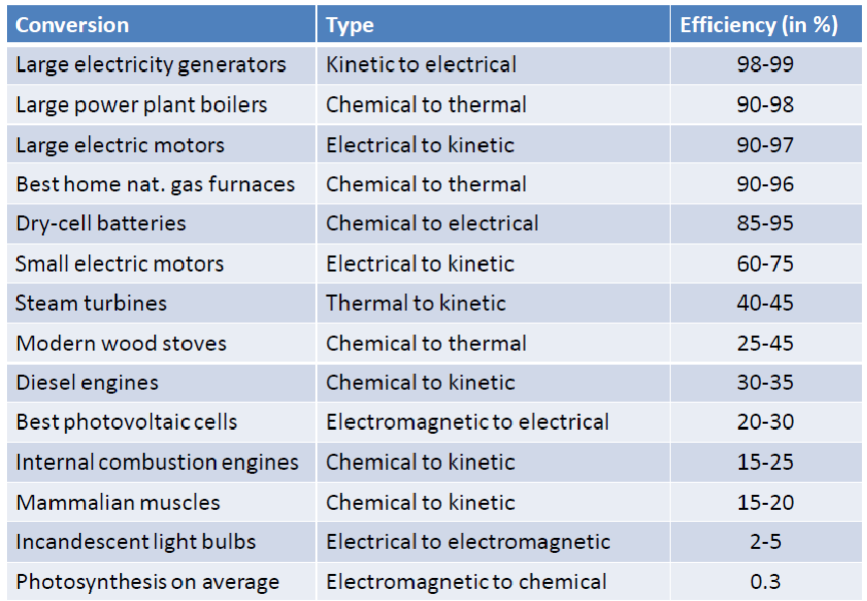
Conclusion:
In conclusion, energy and power are essential concepts in physics and engineering, representing the capacity to do work and the rate at which work is done or energy is transferred, respectively. Understanding these concepts and their various forms is crucial for designing efficient systems for energy generation, conversion, and utilization.