Buffer
Buffers are important for many different reasons. One of the most critical roles of a buffer is when it comes to biology most biochemical processes can only occur when the pH inside the body or cell remains within a fairly narrow range, “An excess of H+ or OH- can interfere with the structure and activity of many biomolecules, especially proteins” [1].
The purpose of this experiment was to determine the effectiveness of different solutions to resist changes of pH. To bring this experiment six different solutions were made. The solutions made during this experiment are listed below.
Solution #1: 50 mL of 0.05M acetic acid and 0.205 g of sodium acetate
Solution #2: 25 mL of 0.05 M acetic acid, 25 mL of DI water, and 0.205 g of sodium acetate
Solution #3: 50 mL of 0.05 M acetic acid and 0.102 g of sodium acetate
Solution #4: 50 mL of 0.05 M acetic acid
Solution #5: 50 mL of DI water and 0.205 g of sodium acetate
Solution #6: 50 mL of DI water
The claim for this experiment is that the solutions that have the larger amount of sodium acetate (0.205g) will be better at resisting pH change from HCl. The solutions that were made with greater amounts of (50mL) of acetic acid will be better at resisting pH change from NaOH. The reason that this claim was made is that the more base the solution has the more acid it can counterbalance and vice versa.
After the solutions were then split evenly into 2 beakers. Half of the solution will be subject to acid (HCl) while the other half will be to base (NaOH). Acid and base were added to each of the solutions 1mL at a time until a significant jump in the pH was measured. The results from this experiment are listed
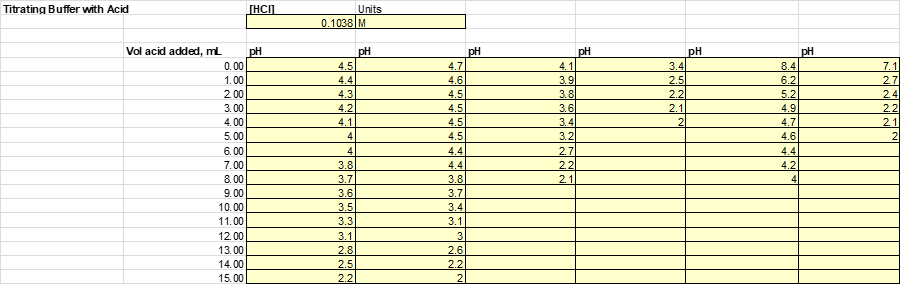
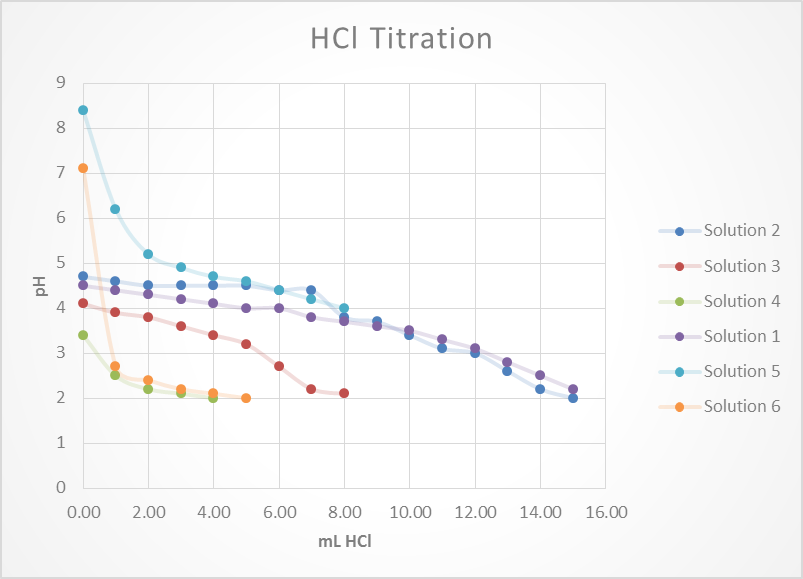
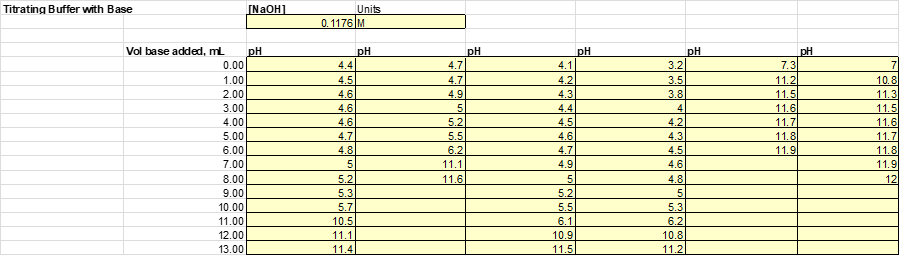
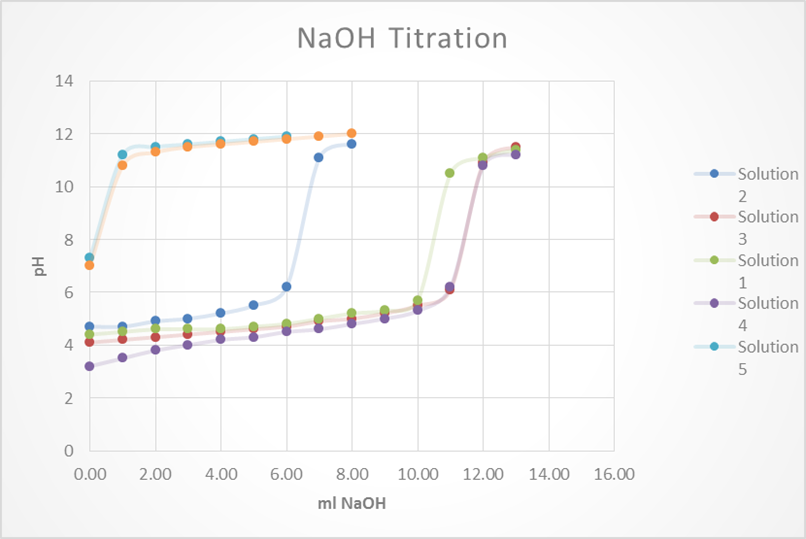
Based on this graph and data tables it is easy to determine which solution was the best buffer as it took the longest for the pH to approach the pH of the titrant. The solutions that were the best buffer for HCl were solution 1 and solution 2. The solutions that were the best buffer for NaOH were solutions 1, 3, and 4. Solution 1 was a good buffer for both HCl and NaOH.
This matches well with the theoretical data as it was calculated that for 0.1M HCl it would take 12.5 mL to titrate solutions 1, 2 & 5. It was also calculated that for 0.1M NaOH it would require 12.5 mL to titrate 1, 3 & 4. As stated before, this does match perfectly with the experimental data. The only difference was that the titration for solution 5 was stopped once it reached a pH of 4 whereas this experiment should have been continued.
Possible sources for error in this lab were that the pH probe only went down to a pH of 2. So when titrating with HCl, one was limited by the abilities of the probe. Another place where were could occur as it could be difficult to measure 1mL of titrant with the burette, so the volume of titrant added to the original solution had room for error.
Reference:
[1] http://www.austincc.edu/biocr/1406/labm/ex3/prelab_3_5.htm