Equilibrium Binary Phase Diagrams
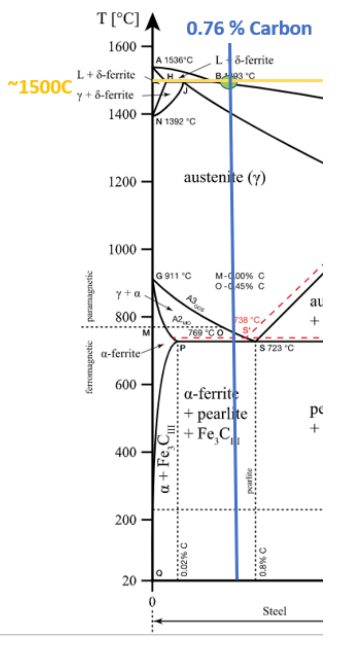
What are the possible phases that can be found on the Iron-Carbon diagram?
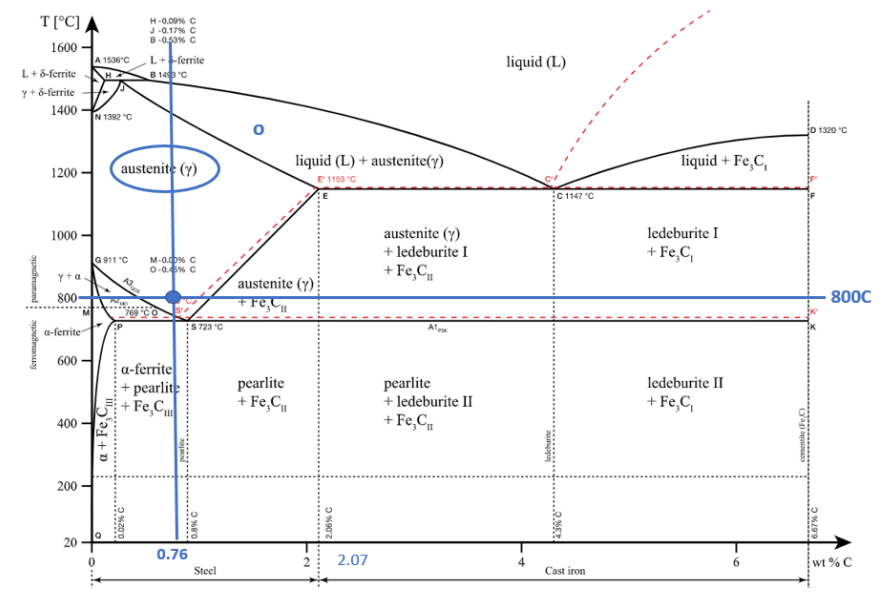
There are three phases which can be found in the Iron-Carbon diagram α (ferrite), γ(austenite), and δ (high temperature ferrite). This iron carbon alloy can have either a face-centered cubic (FCC) lattice, or a body-centered cubic (BCC) lattice, depending on its phase. The compound you will create will depend on the temperate you are at In addition, the carbon content of your solution. If the weight percent of carbon added to the iron is less, then 2.3% you will create steel. If the weight percent of carbon is greater than 2.3% cast iron will be created. These two metals have drastically different properties and that is all due to differences in the composition of the metals. The strength of steel comes from the ceramic cementite (Fe3C), which embeds itself in the iron alloy, giving the steels its strength. However, when the weight percent of carbon goes above 2.5% the cementite starts to break down and graphite starts to be created in cast iron (Fe3C –> C + 3Fe).
What are the eutectic temperature and the eutectic composition? What are the eutectoid temperature and eutectoid composition?
The eutectic point is where a liquid convert into two different solids. This behavior can be observed at the top of the graph where the liquid region converts to austenite + ledeburite + Fe3C and ledeburite+ Fe3C.
Eutectic Temp = 1147 C
Eutectic composition 4.3%
The eutectoid point is when a solid converts into two others solid. This reaction occurs need the middle of the page on the left-hand-side close of the intersection of the austenite region to the ferrite and pearlite regions of the phase diagram.
Eutectoid Temp = 723 C
Eutectoid composition 0.8%
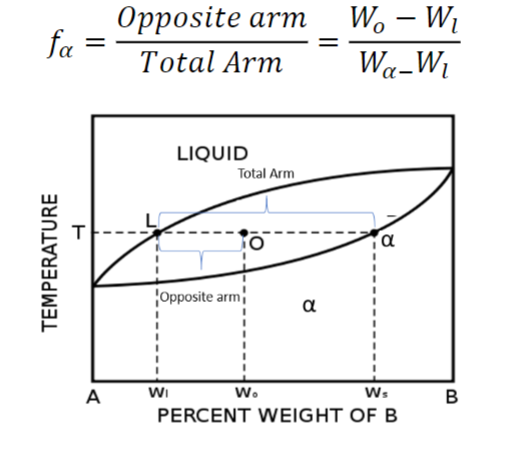
At 1400 C, what is the ratio of liquid to solid for an alloy with a nominal composition of 1.5% C and 98.5% Fe?(use the lever rule)
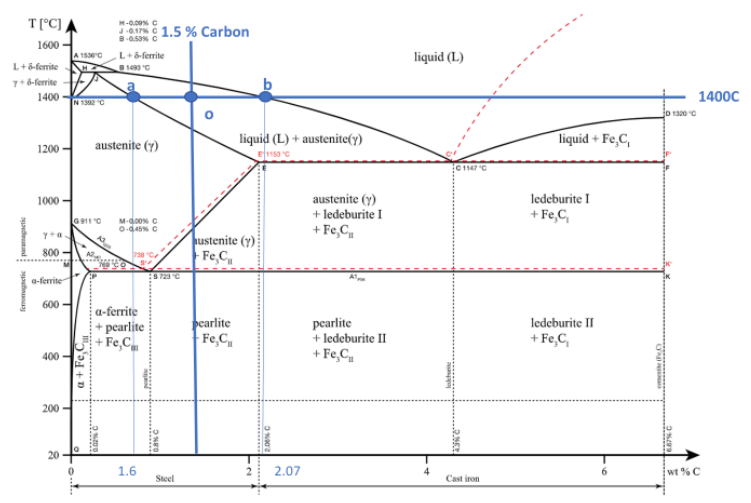
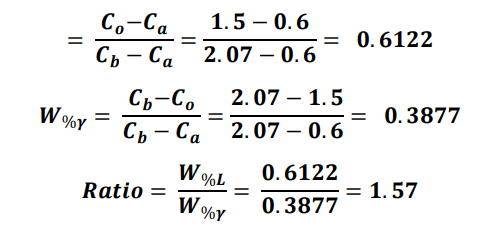
At 800 C, what are the phases present for an alloy with a nominal composition of 0.76% C and 99.24% Fe?
At 800C and 0.76%C the phase present in the alloy will be γ (austenite)
What are the melting temperatures of alloy 2 and 3?
Alloy 2 has a melting point of about 1450C

Alloy 3 has a melting point of about ~1500C