Electric Charge, Fields, and Forces: Key Concepts
Electricity and magnetism form the foundation of countless technologies and natural phenomena. Get ready to delve into the essential concepts of electric charge, fields, and forces. Whether you’re a student or just curious, these principles offer insight into how the invisible forces of electricity shape the world around us.
What is an electric charge simple definition?
Objects can have a property called electric charge (usually just called a charge), which is measured in coulombs (C). The SI unit of electric charge is 1 coulomb = 1 C. An electric charge is a fundamental property of matter that can be: positive (+) and negative (–).
Fundamental Charges and Particles:
| Particle | Charge | Rest Mass |
|---|---|---|
| Electron | −1.602×10−19 C | 9.109×10−31 kg |
| Proton | +1.602×10−19C | 1.673×10-27 kg |
| Neutron | Zero | 1.675×10-27 kg |
Neutral objects have equal amounts of positive and negative charge. An object becomes charged by gaining or losing electrons. Electric charge is responsible for the interaction between objects through electric forces, where like charges repel and opposite charges attract.
Principles of Electric Charge
- Conservation of Charge: The principle of conservation of charge is that the total charge in an isolated system does not change. In other words, electric charge is neither created nor destroyed; it is simply transferred or redistributed “what goes in, must come out” rule for electric charges.
- Quantization of Charge: Charge quantization is the concept that electric charge exists only in discrete, indivisible units. It means that charge cannot be divided into smaller fractions but occurs in multiples of a fundamental charge unit. Charge exists in discrete quantities, typically multiples of the elementary charge (e).
Conductors, Insulators, and Semiconductors
Engineering materials are classified based on their ability to allow charge to flow:
- Conductors: Many free-charge carriers carry current. The conduction band and valence band overlap, allowing the free movement of electrons and resulting in high conductivity. For example, copper and aluminum have about 1029 free electrons/m3.
- Insulators: Few free-charge carriers. A large energy gap exists between the valence band and the conduction band, preventing electrons from moving freely and resulting in low conductivity. Examples include glass and rubber.
- Semiconductors: Moderate charge carriers. There is a small energy gap between the valence band and the conduction band. This allows some electrons to move to the conduction band under certain conditions, providing intermediate conductivity. Examples include silicon, germanium, and gallium arsenide.
It is important to note that conductivity increases with temperature as more electrons gain enough energy to cross the band gap.
What determines whether a material is a conductor semiconductor or insulator?
Materials are broadly categorized as conductors, semiconductors, and insulators based on their conductivity. Those with an electrical conductivity (σ) greater than 105 S/m are classified as conductors, while materials with conductivity below 10−8 S/m are considered insulators. Materials falling within the intermediate range are classified as semiconductors.
Conductivity of metals
Metallic conduction is a fundamental physical property used to distinguish metals from non-metals. Native metals are rare in rocks but hold significant economic value. Among the most notable native metals are copper and gold. Other metals, such as platinum, iridium, osmium, and iron, can also be found in their elemental form, though they are exceedingly rare, particularly at the Earth’s surface. Carbon, often found as graphite, displays unusual behavior: it acts as a metallic conductor along one crystal plane but functions as a semiconductor in the perpendicular direction.
The Drude theory models metal conductivity by treating valence electrons as a free electron gas that moves ceaselessly but produces no net current without an applied electric field. When a field is applied, electrons accelerate but are limited by collisions with atomic nuclei, which occur every 10−13seconds (relaxation time). Conductivity depends on free electron density, electron charge, relaxation time, and effective mass. Perfectly ordered metals would have very high conductivity, but real metals contain crystal imperfections and impurities, reducing their conductivity, especially in alloys.
What is Drude’s formula for metallic conductivity
Drude’s formula for metallic conductivity is expressed as:
σ =ne2 τ / m∗
where σ is electrical conductivity in S/m, n is the number of free electrons per unit volume of the metal, e is the charge of an electron (1.6 ×10−19 coulomb), τ is the scattering time, and m* is the mass of an electron (9.11×10−21 Kg).
What is electric current in simple words?
Electric current is the motion of electric charge through a conductor, such as a wire. The SI unit of electric current is 1 ampere = 1 amp = 1 A: 1 C ≡ 1 A·s (≡ is the mathematical symbol for “is defined to equal”).
There are two kinds of charge, positive (+) and negative (–).
Neutral objects have zero total charge, which may result from equal magnitudes of positive and negative
charges. We charge an object by adding or removing electrically charged particles (usually electrons). Charges of the same sign repel one another. Charges of opposite signs attract one another

What is the Coulomb’s law?
Coulomb’s law is a fundamental principle in physics that describes the electrostatic interaction between electrically charged particles. Coulomb’s law states that the force between two electric charges is directly proportional to the product of their magnitudes and inversely proportional to the square of the distance between them. (The equation below is for two-point charges in vacuum ≈ air)

Where:
- F: Force (N),
- ϵ0: Permittivity of free space (8.854×10−12 C2 / N⋅m2 ),
- q1,q2: Charges (C),
- r: Distance between charges (m). r is never negative.
Key observations:
- Like charges repel; opposite charges attract.
- Force magnitude decreases with the square of the distance. Recall that the
magnitudes of vectors are never negative. - ε0 is a constant:
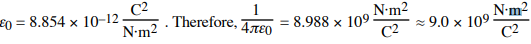
A charged object attracts a neutral one because, since the opposite-sign attracting charges in the two objects are closer together and the same-sign repelling charges are farther apart, there is more attraction than repulsion. For the same reason, the neutral object attracts the charged one with a force that is equal in magnitude but opposite in direction.
Where is Coulomb’s law applied?
Coulomb’s law is specifically applicable to stationary electric charges, as moving charges generate magnetic fields that interact with other forces, making Coulomb’s force insufficient on its own. Despite this limitation, Coulomb’s law plays a crucial role in many areas of physics. Coulomb’s law is crucial in various fields such as electromagnetism, electrodynamics, quantum mechanics, cosmology, and thermodynamics, highlighting its broad applicability and fundamental importance in physics
How does an object of charge q attract or repel an object of charge q0?
- Step 1: The first object, because it has charge q, sets up an electric field E in the space around that object.
- Step 2: The second object, because it has charge q0, experiences a force F0 from that electric field.
What is an electric field in simple terms?
An electric field (E) represents the force experienced by a unit charge in space. An electric field is a region around an electric charge or collection of charges where a force can be exerted on other charged objects. It describes the influence or effect that the charge has on its surroundings, causing electrically charged particles to experience a force when placed in the field.

We define the electric field (E) at any point to be the electric force per charge F0/q0 on a test charge q0 placed at that point: E is in N/C Thus vector equations are
and
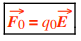
where E is an external electric field. (That is, E is not the electric field of q0 itself.) These vector equations tell us that E and F0 are in the same direction if q0 is positive (+), but E and F0 are in opposite directions if q0 is negative (–). To find the magnitude E of the electric field of a point charge of absolute charge value |q|.
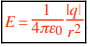
If the charge q is positive (+), the electric field E it sets up is away from it, but if the charge q is negative (–), the electric field E it sets up is toward it. The total electric field at any point is the vector sum of the individual electric fields at that point.
Electric Field Lines
- Field lines are visual tools to represent electric fields:
- Direction: Tangent to the line at any point.
- Density: Greater where the field is stronger.
- Start on positive charges and end on negative charges.
Conclusion
In conclusion, the world of electric and magnetic forces is truly astonishing and holds many shocking truths. From the fascinating interactions of electric charges to the invisible power of magnets, these forces shape our daily lives in ways we might not even realize. The conservation of charge, the behavior of conductors, semiconductors, and insulators, and the quantization of charge all contribute to our understanding of how these forces operate. Moreover, Coulomb’s law provides us with a mathematical foundation to comprehend the strength of these forces in various scenarios.
Electricity and magnetism are not just concepts confined to physics textbooks; they are an integral part of the technological marvels that surround us. From electrical appliances to advanced medical devices, our world owes a tremendous debt to these forces. Understanding electromagnetism has revolutionized industries and communication, bringing about an era of technological advancement.
Practical Applications
These concepts are fundamental in understanding:
- Electrical circuits and components (conductors and insulators).
- Forces between charged objects, are essential in sensors and particle physics.
- Field interactions, form the basis of technologies like capacitors and electric motors.