The Hydrogen Car
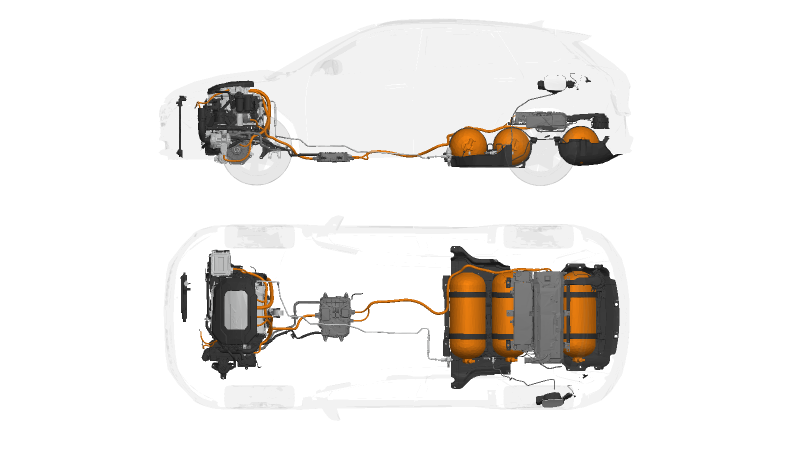
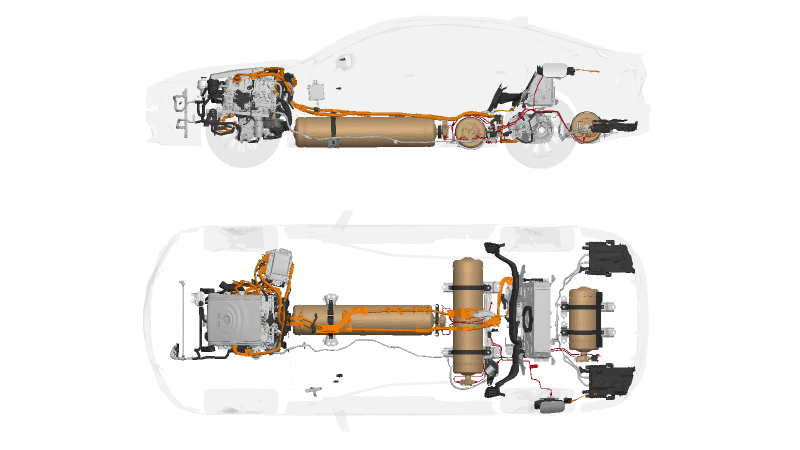
Similar to all-electric vehicles (EVs) a Hydrogen Car/Fuel cell electric vehicle (FCEV), utilizes electricity to drive an electric motor. However, unlike other EVs, FCEVs generate electricity using a hydrogen-powered fuel cell instead of relying on a battery for power. While FCEVs could be designed with plug-in capabilities similar to PHEVs to charge the battery. Most current FCEVs primarily use the battery for regenerative braking, providing additional power for short bursts of acceleration and stabilizing the power output from the fuel cell.

This allows the option to idle or shut off the fuel cell when power demands are low. The storage capacity of energy on board is determined by the size of the hydrogen fuel tank, which differs from EVs, where power and energy availability are closely tied to the battery’s size.
Hydrogen Cars and the Automotive Industry
Most major auto manufacturers are actively engaged in researching and working on developing fuel cell vehicle projects by exploring various fuel cell and hybrid combinations that include conventional combustion, fuel reformers, and battery power. While hydrogen fuel offers greater efficiency compared to gasoline, it is also approximately four times more costly to refuel.
In May 2024, Toyota established its North American hydrogen business headquarters in Los Angeles, known as H2HQ. This facility will focus on R&D, commercialization, and sales of hydrogen fuel cell products across various sectors, including passenger cars, heavy-duty trucks, stationary fuel cell power generation, and port vehicle applications. In 2023, Toyota established Hydrogen Factories in Japan and Europe to oversee businesses in those continents
Currently, there are approximately 17,000 hydrogen-powered vehicles in operation in the United States, all of which are located in California. In contrast, there are millions of EVs on the roads. While EVs are receiving significant attention, hydrogen vehicles are still largely in the background. This article provides an overview of what hydrogen cars are, how they operate, and the likelihood of them becoming a viable option for consumers.
Is there a car that runs on hydrogen?
Since 2015, three car companies have introduced hydrogen-powered cars for sale: the Honda Clarity Fuel Cell, the Hyundai Nexo SUV, and the Toyota Mirai. However, Honda has ended production of all Clarity models. Hyundai has sold only approximately 1600 Nexo SUVs over six years. Toyota, which has shown the most commitment to FCEVs, has sold around 14,300 Mirai over its two generations in the U.S.
To sell these the last couple of Mirai its dealer lots, in 2024 Toyota had to provide customers with a 60% discount on its Mirai. Additionally, the automaker also offered its customers $15,000 worth of complimentary H2 fuel and interest-free financing.
This recent incentive from Toyota could be seen as an effort to clear out the remaining inventory promptly. Previously, the automaker had provided a $32,000 discount on its 2020 Mirai model in early 2021, before the launch of the 2021 version of the car.
General Motors: Hydrogen Car Prototype
General Motors’ GMC S-10, introduced in 2001, was a FCEV that ran on low-sulfur gasoline fuel. It featured a 25 kW PEM (Proton Exchange Membrane) fuel cell, achieving an impressive 40 miles per gallon and a 112 km/h top speed.

Is GM working on hydrogen fuel cells?
GM will supply the first medium-duty truck powered by hydrogen fuel cells to a utility company in Georgia. This is part of a federally funded project to decarbonize the transportation sector. The truck, based on the 2024 Chevrolet Silverado 5500, will have a GM-estimated range of over 300 miles and a gross vehicle weight rating of 19,500 lbs. It will also generate over 300 kW of power for off-board use on job sites.

What makes this so exciting?
GM and Honda have initiated commercial production at the industry’s first hydrogen fuel cell system manufacturing joint venture. This development marks a crucial milestone in commercializing hydrogen fuel cell systems Fuel Cell System Manufacturing, LLC (FCSM).
FCSM marks a significant milestone as the first large-scale manufacturing joint venture dedicated to producing fuel cells. By Leveraging the intellectual property and expertise of both GM and Honda, the FCSM team has successfully developed more affordable and commercially viable hydrogen fuel cell systems. These systems are intended for use in a range of zero-emissions propulsion and energy management applications, including Renewable Innovations’ mobile power generators and Autocar’s class 7 and 8 trucks.
Ford: Hydrogen Car Prototype
Ford’s Advanced Focus FCV, introduced in 2002, represented another milestone in fuel cell vehicle technology. This model was a fuel cell battery hybrid, featuring an 85 kW PEM (Proton Exchange Membrane) fuel cell. It achieved an approximate equivalent of 50 miles per gallon, showcasing the efficiency of hydrogen fuel cells. The car stored its fuel in 4 kg of compressed hydrogen gas at 5000 psi, a common method for storing hydrogen in vehicles.

Daimler-Chrysler: Hydrogen Car Prototype
Daimler-Chrysler’s NECAR 5, unveiled in 2000, was a groundbreaking fuel cell vehicle that pushed the boundaries of hydrogen technology. This model featured an 85 kW PEM fuel cell and ran on methanol fuel, requiring a reformer to convert the methanol into hydrogen for the fuel cell. With a top speed of 150 km/h, the NECAR 5 demonstrated that fuel cell vehicles could achieve high-performance levels.

A later version, the NECAR 5.2, completed an impressive drive from California to Washington DC, showcasing its long-distance capabilities. The NECAR 5.2 was awarded a road permit for Japanese roads, highlighting its international recognition and the growing acceptance of fuel cell vehicles in global markets.
Mitsubishi: Hydrogen Car Prototype
The Mitsubishi Grandis FCV minivan represented a significant step forward in fuel cell vehicle design. Introduced as a fuel cell/battery hybrid, it featured a 68 kW PEM fuel cell. The vehicle used compressed hydrogen fuel, a common method in fuel cell vehicles. Despite its size, the Grandis FCV boasted a respectable top speed of 140 km/h, showcasing the potential for fuel cell technology in larger vehicles like minivans.

Are Hydrogen Cars Safe?
Hydrogen cars are generally regarded as safe as conventional cars. The high-pressure tanks are engineered to withstand even the most severe collisions without leaking or rupturing. Despite occasional references to the Hindenburg disaster of 1937 by hydrogen skeptics. The hydrogen tanks and associated hardware in hydrogen cars would remain intact even if the rest of the vehicles were severely damaged in an accident. To date, no injuries or fatalities related to the hydrogen components have been reported in the relatively small number of hydrogen cars sold.
Are hydrogen cars better than electric vehicles?
When comparing hydrogen cars to electric vehicles (EVs), battery electric vehicles emerge as the clear winner, primarily due to their popularity and the more well-established infrastructure to support them. EV ranges typically vary from approximately 100 miles to about 350 miles. In contrast, hydrogen cars offer an extended driving range and quicker refueling times. The range of a hydrogen vehicle can span from 400 to 600 miles, contingent upon the size of the tank.
In the United States, there are just 52 hydrogen stations accessible to the public, with all but one located in California. The remaining station is in Hawaii. Unlike EV charging stations, which often feature multiple ports, most hydrogen stations have only a single outlet, allowing only one vehicle to refuel at a time.
Finally, hydrogen cars are not equipped with large batteries capable of delivering significant power surges to their motors. As a result, they do not offer the strong acceleration typical of most EVs. Instead, they are better suited for comfortable cruising rather than high-performance driving.
Fuel Cell Vs. Battery
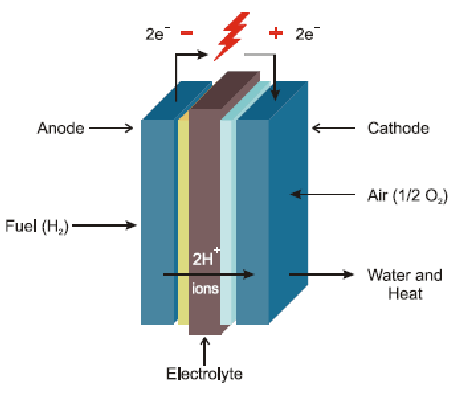
The basic operating principles of fuel cells and galvanic batteries are quite similar, but there are several
intrinsic differences between the two. A hydrogen fuel cell operates as an open system, with the anode
and cathode being gases in contact with a platinum catalyst. The reactants, such as hydrogen and
oxygen, are externally supplied, and the cell does not require recharging; it generates electricity as long
as fuel is provided.
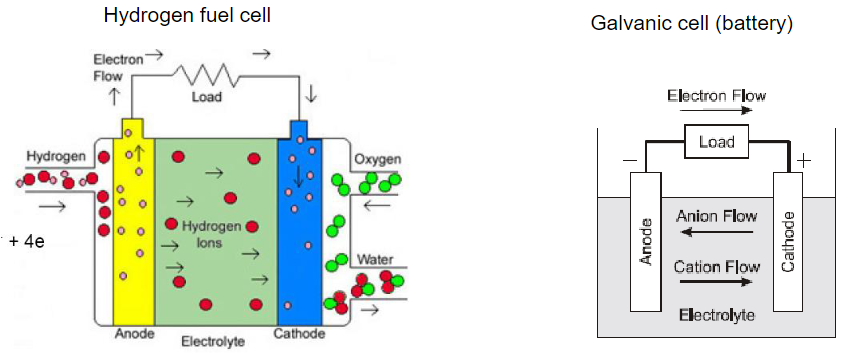
On the other hand, a galvanic cell, or battery, operates as a closed system. The anode
and cathode are typically metals, and the reactants are internally consumed during the chemical
reaction. This means that galvanic cells need periodic recharging to restore the reactants and maintain
their ability to generate electricity.
Fuel Cell Vs. Internal Combustion Engine
Fuel cells and internal combustion engines share some similarities despite their distinct modes of operation. Both systems utilize hydrogen-rich fuels and compressed air as the oxidants for their respective processes. Additionally, they both require cooling to manage heat generated during operation.
However, their outputs and pollution levels differ significantly. Fuel cells produce electrical work through an electrochemical reaction between the fuel and oxidant, resulting in little to no pollution. In contrast, internal combustion engines generate mechanical work through a combustive reaction between fuel and oxidant, typically fossil fuels, which can lead to significant pollution emissions.
Fuel Cells in Use: Transportation Systems
One of the most commercially advanced applications of fuel cells to date in transportation systems is in buses. Many American and European cities are currently using fuel-cell buses as part of their public transportation fleets. These buses offer an environmentally friendly alternative to traditional diesel buses, as they produce zero emissions at the tailpipe. The use of fuel cells in buses demonstrates the feasibility and effectiveness of this technology in reducing greenhouse gas emissions and improving air quality in urban areas.

Barriers to Fuel Cell Adoption
The adoption of fuel cells faces several barriers, primarily due to the high costs associated with key components. Pt-based electrocatalysts, used in fuel cell electrodes, are particularly expensive, with Pt priced at around $1530 per troy ounce. This translates to approximately $100 per kilowatt (kW) for a fuel cell with 1.0 mg/cm² Pt loading and a power density of 0.5 W/cm².
For example, a 100-horsepower (HP) fuel cell would require 75 kW, costing $7500 for the catalyst alone. Additionally, the proton exchange membrane, crucial for ion transport within the fuel cell, is costly, with a perfluorosulfonic acid (PFSA) membrane priced at $400 per square meter, totaling $6000 for a 100 HP fuel cell.
Efforts are underway to reduce costs by reducing catalyst loading and exploring alternatives to Pt, such as palladium (Pd) and ruthenium (Ru), which are less expensive. There are also initiatives to develop lower-cost membranes, with projections suggesting PFSA membrane costs could fall below $50 per square meter at high-volume production, along with the use of non-fluorinated membranes. Additionally, ensuring a stable and affordable fuel supply remains a challenge for widespread fuel cell adoption.
Conclusion
In conclusion, hydrogen cars offer a promising glimpse into the future of sustainable transportation. Despite their current challenges, such as limited infrastructure and higher costs compared to traditional vehicles, they provide several advantages, including longer driving ranges and shorter refueling times. As technology continues to evolve and infrastructure expands, hydrogen cars could become a viable and environmentally friendly alternative to conventional vehicles. With ongoing advancements and increasing global interest in renewable energy, hydrogen cars have the potential to play a significant role in reducing carbon emissions and shaping a more sustainable automotive industry.