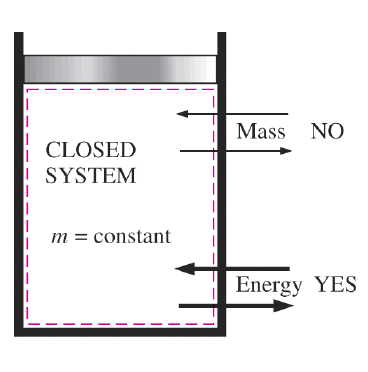
Understanding Availability and Exergy in Thermodynamics
In the world of thermodynamics, analyzing energy alone is not enough to fully understand how systems perform or how efficiently they operate. While energy is conserved, not all energy is equally useful, this is where the concepts of availability and exergy come into play.
These two ideas provide insight into the quality of energy and the real potential for doing work within a system. Rooted in the second law of thermodynamics, exergy helps identify inefficiencies, quantify losses, and guide improvements in engineering processes. Whether in power generation, refrigeration, or industrial manufacturing, understanding availability and exergy is essential for designing functional, efficient, and sustainable systems. This article explores these key thermodynamic concepts, their significance, and how they are applied to optimize performance and reduce waste in real-world systems.
Defining Availability and Exergy
Exergy is the maximum amount of useful work that can be extracted from a system as it moves toward equilibrium with a reference environment. It represents the theoretical limit of a system’s ability to do work. In contrast, availability refers to the exergy of a system at a given state, relative to a specific environment.
Exergy is particularly valuable because it provides a measure of energy quality, distinguishing between energy that can perform work and energy that is irreversibly lost due to entropy generation.
Why are exergy and entropy generation important in the design of a thermal system?
It is crucial to assess the available energy remaining at the end of a process, known as exergy, and how efficiently that energy is used, which is indicated by entropy generation. Both of these concepts are derived from the second law of thermodynamics. Accurately predicting these parameters is essential for the efficient design and development of heat exchangers and other thermal systems, as they directly influence performance and optimization.

What is the difference between energy and exergy?
A system that is in complete equilibrium with its surroundings possesses no exergy. In such a state, there are no differences in temperature, pressure, or concentration, meaning there is no driving force for any process to occur.
The exergy of a system increases as it becomes more different from its environment. For example, a given amount of hot water has a higher exergy content in winter than in summer, due to the greater temperature difference. Similarly, a block of ice may have minimal exergy in cold weather but can possess significant exergy on a hot day.

As energy loses its quality through irreversible processes, exergy is destroyed. Exergy represents the usable portion of energy, the part capable of doing work, and thus holds economic value, making its management particularly important.
By definition, exergy depends not only on the state of the system or energy flow but also on the conditions of the surrounding environment.
Exergy efficiency measures how closely a process approaches ideal or reversible behavior. Unlike energy efficiency, which can sometimes be misleading, exergy efficiency provides a more accurate reflection of system performance.
A Simplified Approach to Energy and Exergy Analyses
To ensure clarity, consistency, and verifiability in the evaluation of any process, system, or application, it is important to follow a standardized procedure when conducting energy and exergy analyses. This not only makes the results easier to understand and review by experts in the field but also provides a framework for benchmarking methods and outcomes. A streamlined approach to these analyses generally includes the following steps:
- Divide the overall process into smaller, manageable sections based on the desired level of detail and insight.
- Conduct standard mass and energy balances for each section, determining key quantities such as heat and work, along with system properties like temperature and pressure.
- Choose an appropriate reference environment model based on the type of process, the acceptable level of complexity and accuracy, and the specific goals of the analysis.
- Calculate energy and exergy values relative to the chosen reference environment.
- Perform exergy balances, including assessments of exergy destruction and losses.
- Define efficiency metrics based on the objectives of the analysis, and compute the corresponding efficiency values.
- Analyze and interpret the results, forming conclusions and recommendations regarding potential design optimizations, system improvements, or retrofit strategies.
This structured methodology provides a clear and effective path to assess performance and identify areas for thermodynamic improvement.
Energy and Exergy Efficiencies
Efficiency plays a critical role in evaluating resource utilization and system performance, especially in engineering applications like power plants, heaters, refrigerators, and thermal storage systems. Traditionally, energy efficiency, based on the first law of thermodynamics, is used to assess how much energy input is converted into useful output. However, this measure often falls short in accurately reflecting the true performance of a system, as it does not consider the quality of energy or the irreversibilities that occur within the process.
In contrast, exergy efficiency, grounded in the second law of thermodynamics, provides a more realistic and insightful metric. It not only evaluates the ratio of useful exergy output to total exergy input but also identifies the locations, causes, and magnitudes of inefficiencies. Unlike energy analysis, which can sometimes misrepresent where losses occur or suggest unrealistic efficiencies (e.g., values greater than 100% in COPs), exergy analysis keeps results within a rational 0% to 100% range.
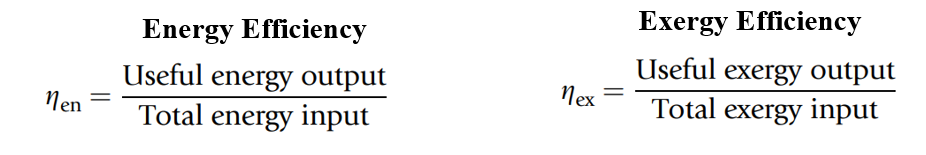
It distinguishes between losses due to irreversibilities and those associated with waste streams, offering a clearer picture of where improvements are possible. Thus, while both energy and exergy efficiencies are useful, exergy efficiency ultimately delivers a more comprehensive and meaningful understanding of system performance and its potential for optimization.
What is availability in thermodynamics?
The maximum amount of useful work that can be extracted from a heat source is known as its availability or exergy. The portion of energy that must inevitably be rejected, as dictated by the second law of thermodynamics, is referred to as energy. The availability also declines as the temperature difference between a system and its surroundings decreases.
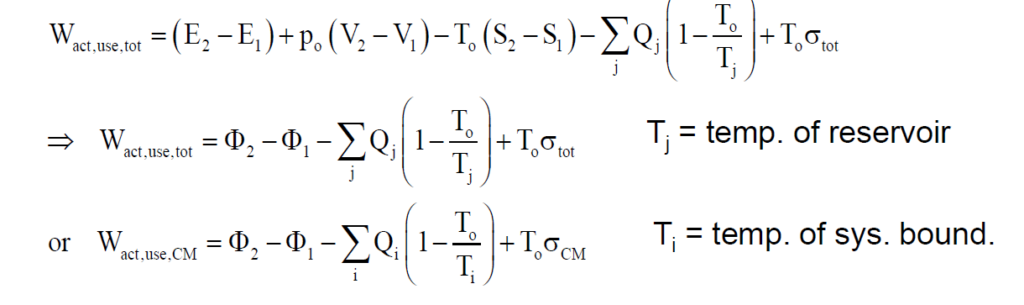
What is the availability function for a closed system?
The availability of a system is defined as the maximum amount of useful work that can be produced during a process in which the system reaches equilibrium with its surroundings, also known as the dead state. The availability function for a non-flow process, or a closed system, is expressed as:
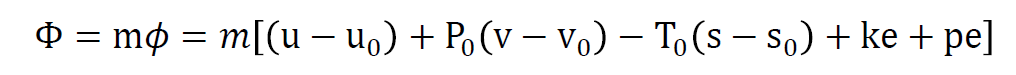
Conclusion
Understanding availability and exergy provides a deeper, more meaningful perspective on energy use in thermodynamic systems. Unlike traditional energy analysis, which focuses solely on quantity, exergy highlights the quality of energy and the real potential for useful work.
It reveals where inefficiencies occur, why, and how they can be minimized. By incorporating the principles of the second law of thermodynamics, engineers and researchers can design more effective, sustainable, and economically viable systems from power plants and refrigeration units to complex industrial processes. As the global demand for energy efficiency and environmental responsibility continues to rise, mastering the concepts of availability and exergy is not just valuable, it’s essential.