Equilibrium Binary Phase Diagrams
Phase diagrams are crucial for understanding the behavior of elements, compounds, and solutions. They offer valuable insights into phase composition and stability based on temperature (T), pressure (P), and composition (C). Additionally, phase diagrams enable us to study and manage key processes. For example, they can be used to understand phase separation, solidification, sintering, purification, and the growth /doping of single crystals. While phase diagrams primarily provide information about systems at equilibrium, they also help predict phase relations, compositional changes, and structures in non-equilibrium systems.
What is a binary phase equilibrium diagram?
A binary phase diagram, also known as an equilibrium diagram, represents the temperature-composition relationship of a two-component system at constant pressure. In this diagram, the temperature is displayed on the vertical axis, while the composition is shown on the horizontal axis.
What is the phase rule of phase equilibrium?
The phase rule is given by F = C − P + 2. For a single-component system with one phase, there are two degrees of freedom, meaning that both temperature and pressure can be independently varied within certain limits.
How equilibrium conditions are represented in a phase diagram?
These curves depict the connections between phase-transition temperatures and pressures. The intersection of all three curves corresponds to the substance’s triple point. Which is the specific temperature and pressure where all three phases coexist in equilibrium.
What are the possible phases that can be found on the Iron-Carbon diagram?
Three phases can be found in the Iron-Carbon diagram α (ferrite), γ(austenite), and δ (high temperature ferrite). This iron-carbon alloy can have either a face-centered cubic (FCC) lattice, or a body-centered cubic (BCC) lattice, depending on its phase. The compound you will create will depend on the temperature you are at and the carbon content of your solution. If the weight percent of carbon added to the iron is less, than 2.3% you will create steel.
If the weight percent of carbon is greater than 2.3% cast iron will be created. These two metals have drastically different properties and that is all due to differences in the composition of the metals. The strength of steel comes from the ceramic cementite (Fe3C), which embeds itself in the iron alloy. This gives the steel its strength. However, when the weight percent of carbon goes above 2.5% the cementite starts to break down. As a result, graphite starts to be created in cast iron (Fe3C –> C + 3Fe).
What are the eutectic temperature and the eutectic composition? What are the eutectoid temperature and eutectoid composition?
The eutectic point is where a liquid converts into two different solids. This behavior can be observed at the top of the graph where the liquid region converts to austenite + ledeburite + Fe3C and ledeburite+ Fe3C.
Eutectic Temp = 1147 C
Eutectic composition 4.3%
The eutectoid point is when a solid converts into two other solids. This reaction occurs need the middle of the page on the left-hand-side close of the intersection of the austenite region to the ferrite and pearlite regions of the phase diagram.
Eutectoid Temp = 723 C
Eutectoid composition 0.8%
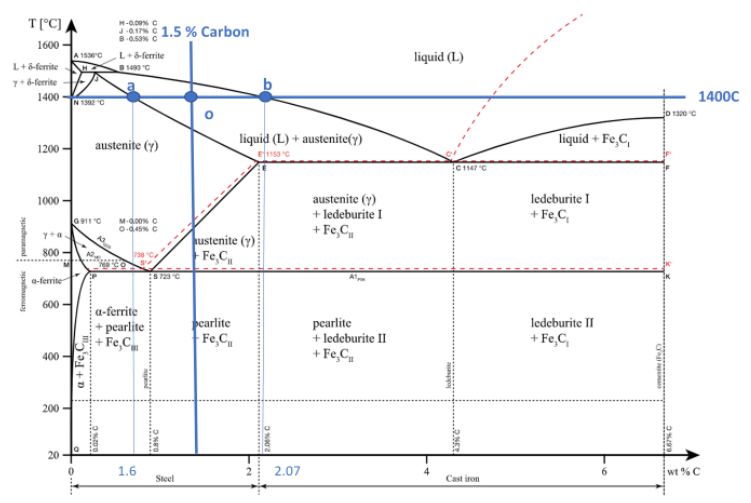
At 1400 C, what is the ratio of liquid to solid for an alloy with a nominal composition of 1.5% C and 98.5% Fe?(use the lever rule)
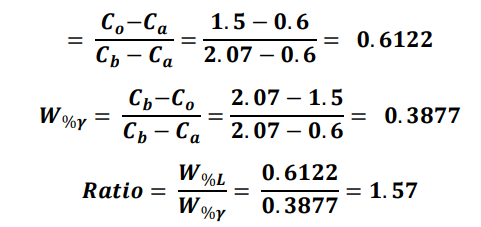
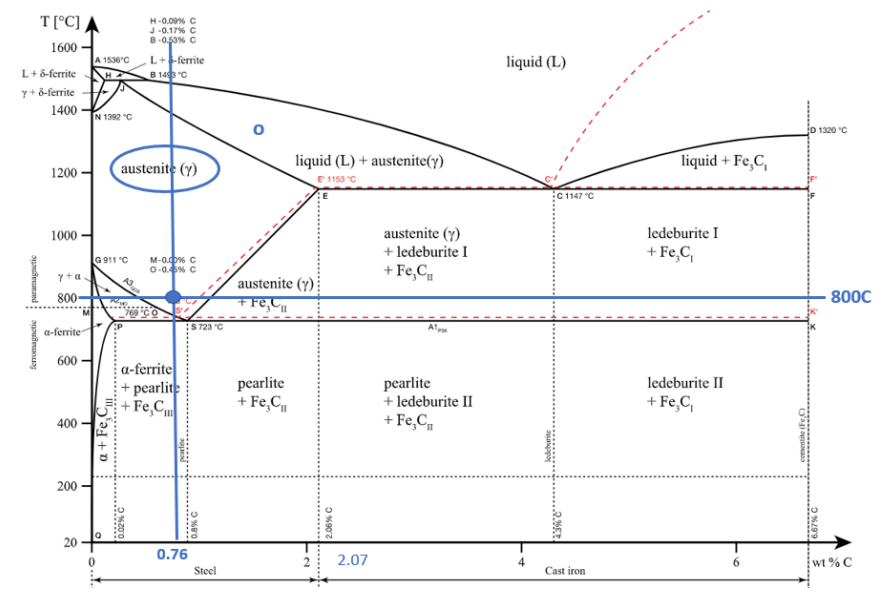
At 800 C, what are the phases present for an alloy with a nominal composition of 0.76% C and 99.24% Fe?
At 800C and 0.76%C the phase present in the alloy will be γ (austenite)
What are the melting temperatures of alloys 2 and 3?
Alloy 2 has a melting point of about 1450C
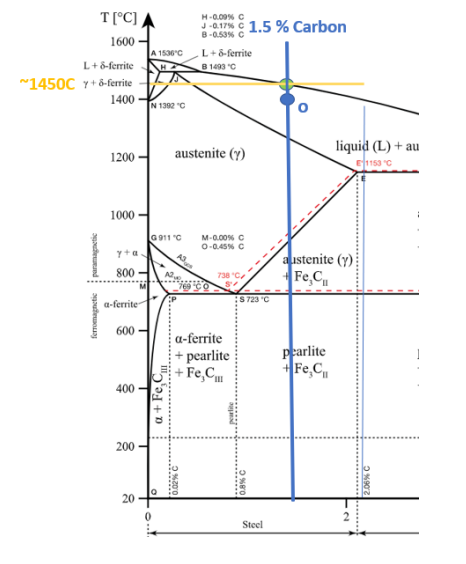
Alloy 3 has a melting point of about ~1500C

Conclusion
In conclusion, equilibrium binary phase diagrams provide a valuable framework for understanding the complex interplay of temperature, pressure, and composition in two-component systems. They serve as indispensable tools for predicting phase behavior, designing processes, and optimizing material properties across a wide range of industries. By elucidating the relationships between phases and their stability, these diagrams continue to play a pivotal role in advancing our knowledge of materials science and engineering.