Lead Acid Car Battery
Conventional vehicles typically rely on Lead Acid Car Battery due to their high power output and affordability. These batteries use water-based electrolytes and have individual cell voltages that are relatively low. While they offer proven safety, lead-acid batteries have a lower specific energy compared to lithium-ion types. In contrast, hybrid electric vehicles often use nickel-metal hydride (NiMH) batteries because of their long lifespan and ability to undergo many charge/discharge cycles.

What is a lead acid car battery?
A lead acid battery is rechargeable and operates using lead and sulfuric acid. The lead is immersed in the sulfuric acid, facilitating a controlled chemical reaction that generates electricity.
Lead acid Battery History
In 1801, French scientist Nicolas Gautherot observed that wires used in electrolysis experiments could produce a small “secondary” current after the main battery was disconnected. In 1859, Gaston Planté invented the first rechargeable lead-acid battery by passing a reverse current through it. The 1970s saw the development of valve-regulated lead-acid (VRLA) batteries.
Major comparative features
Lead-acid car batteries are known for their high discharge rate, making them ideal starter batteries for automobiles. They are typically aqueous or unsealed, requiring low maintenance, with some variants like VRLA (valve-regulated lead-acid) batteries. VRLA batteries are prevalent due to their deep-discharge capability, often used in applications like golf carts. These batteries are commonly referred to as SLI (starting, lighting, and ignition) batteries, reflecting their primary functions in a vehicle.
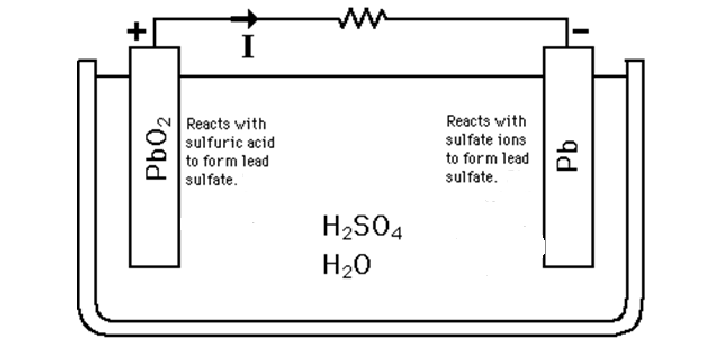
Active circuit parts of a Pb-acid battery cell
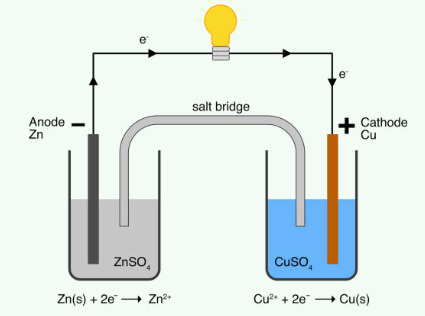
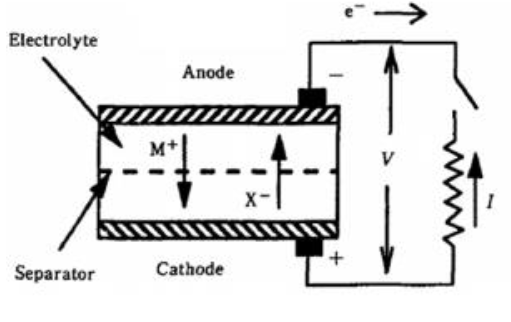
The active circuit parts of a lead-acid battery cell include several components. The negative electrode is made of lead (Pb), while the positive electrode is made of lead dioxide (PbO2). The electrolyte used is typically a 5M H2SO4 aqueous solution. A porous glass mat filled with electrolytes acts as a separator between the two electrodes. Both the negative and positive electrodes are connected to current collectors, which allow the flow of electrons in and out of the cell during the charging and discharging processes.
Typical Lead acid car battery parameters
Typical parameters for a Lead Acid Car Battery include a specific energy range of 33–42 Wh/kg and an energy density of 60–110 Wh/L. The specific power of these batteries is around 180 W/kg, and their charge/discharge efficiency varies from 50% to 95%. Lead-acid batteries have a self-discharge rate of 3–20% per month and can endure approximately 500–800 charge/discharge cycles. The nominal cell voltage for these batteries is 2.0 V, and they can be charged within a temperature range of -35°C to 45°C.

Lead Acid Car Battery – Chemistry
Overall: PbO2+Pb+2H2SO4 →2PbSO4 + 2H2O
Discharging
Negative Plate: Pb + SO42 –→ PbSO4 + 2e–
Positive Plate: PbO2 + SO4 2-+ 4H+ + 2e– → PbSO4 + 2H2O
Charging
Negative Plate (reduction): PbSO4 + 2e– → Pb + SO42 –
Positive Plate (oxidation): PbSO4 + 2H2O→ PbO2 + SO4 2- + 4H++ 2e–
Attention!! “Negative electrode is always losing electrons (oxidation) while Positive electrode always
gains electrons (reduction)” is only true at discharging. When charging, the chemical reaction is reversed.
Why is the car battery 12V?
The car battery is typically 12V because it is made up of six 2V battery cells connected in series. Historically, cars used 6V electrical systems and batteries until the mid-1950s. The shift to 12V systems occurred when larger engines with higher compression ratios required more electrical power to start. This change to 12 volts offered the advantage of requiring less copper to transfer power throughout the vehicle. Additionally, advancements in battery technology enabled the creation of 12-volt batteries that were the same size as the previous 6-volt batteries, making the transition feasible without major redesigns.
Why are battery cells 2 volts?
Because the electric potential (voltage) produced by most chemical reactions is around 2V, which is often insufficient for most loads, many battery cells are connected in series in most batteries. For instance, each cell in lead-acid batteries typically generates a voltage of approximately 2V.
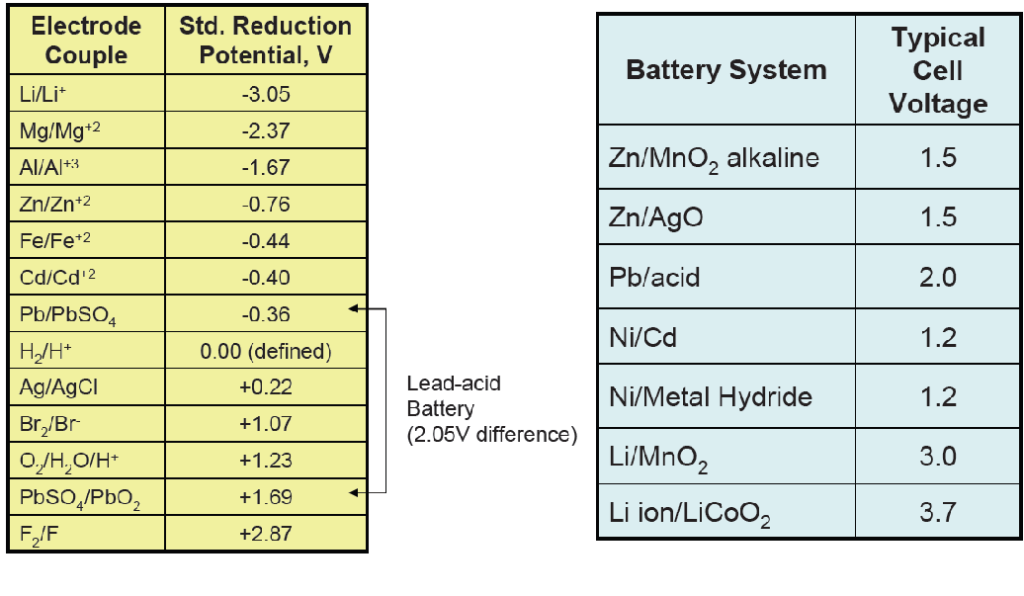
Example Problem: Calculate the OCV. for a battery cell: Lead and lead oxide in sulfuric acid
OCV = Vpositive electrode – Vnegative electrode
Positive electrode: PbO2
Negative electrode: Pb
Vpositive electrode = +1.69 V
Vnegative electrode = – 0.36 V
OCV = + 1.69 V – (– 0.36 V) = 2.05 V
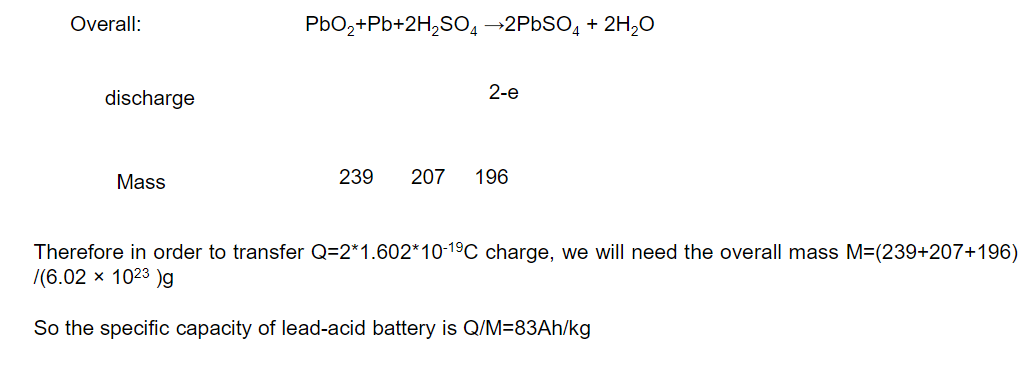
Example Problem: What’s the theoretical specific capacity (Ah/kg) for a Lead Acid Car Battery
Relative mass:
Pb: 207.2
O:16
S: 32.07
Other info:
1. One electron: -1.602*10-19C
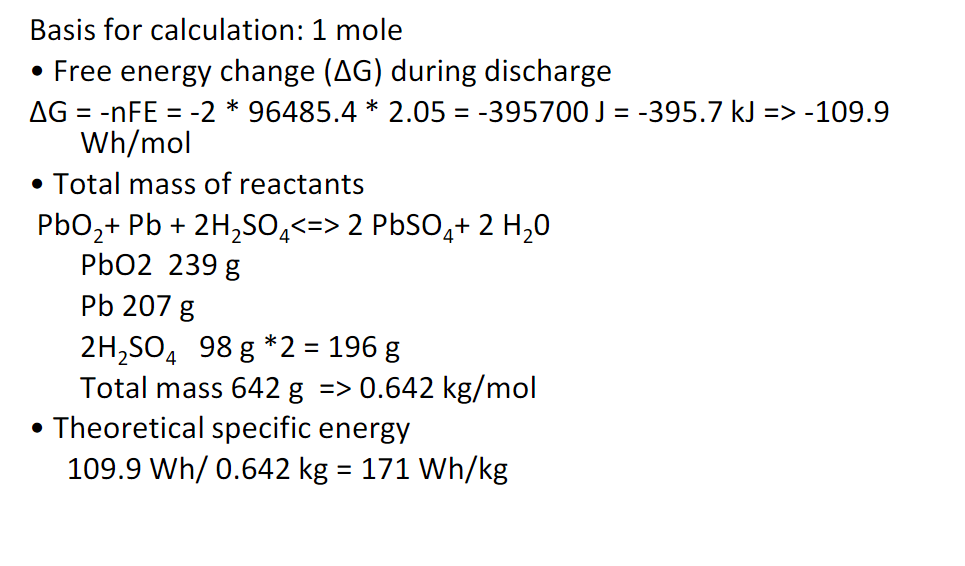
Example Problem: What’s the theoretical specific energy (Wh/kg) for a Lead Acid Car Battery
The specific capacity of a lead-acid battery is 83Ah/kg
The specific energy of the lead-acid battery is 2.05V*83Ah/kg=171Wh/kg
Why are we still using Lead Acid Car Battery?
Even with the ongoing advancement of new battery technologies, Lead acid car batteries remain extensively utilized in the automotive industry. Lead acid car batteries are still widely used due to several advantages. They are the lowest-cost option among battery technologies. The technology is mature, with a well-established worldwide infrastructure for manufacturing and recycling. Lead-acid batteries have a high specific heat, making them safe, especially because they use an aqueous electrolyte. They also have a low self-discharge rate.

However, there are disadvantages to consider. Lead-acid batteries have a low specific energy storage capacity, typically less than 40 Wh/kg. If designed for high power output, they may have poor cycle life when subjected to deep discharges. Performance also degrades significantly at low temperatures. Additionally, lead (Pb) is toxic, which raises environmental concerns, especially regarding disposal and recycling.
Lead Acid Battery Application
Most of the world’s lead-acid batteries are used as automobile starting, lighting, and ignition (SLI) batteries, with an estimated 320 million units shipped in 1999. In 1992, around 3 million tons of lead were used in battery manufacturing.
Lead acid batteries power golf carts, as well as the electric motors in diesel-electric submarines when submerged and serve as emergency power in nuclear submarines. Valve-regulated lead-acid batteries, which cannot spill their electrolyte, are used in backup power supplies for alarm systems, smaller computer systems, uninterruptible power supplies, electric scooters, electric wheelchairs, electrified bicycles, marine applications, battery electric vehicles, micro-hybrid vehicles, motorcycles, and electric forklifts, where their weight serves as a counterweight.
Additionally, lead-acid batteries were historically used to supply filament voltage in early vacuum tube radios and in portable batteries for miners’ cap headlamps, which typically have two or three cells.
Conclusion
In conclusion, despite the emergence of new battery technologies, lead acid car batteries, continue to play a vital role, in various applications, particularly in the automotive industry. Their low cost, mature technology, and reliable performance make them a preferred choice for many. While they do have limitations such as low specific energy, poor performance at low temperatures, and environmental concerns due to lead toxicity, their benefits often outweigh these drawbacks. As we move forward, ongoing advancements and improvements in lead-acid battery technology will likely ensure their relevance and continued use in the years to come.