Lithium Battery
Few technologies have had a larger impact on our everyday lives compared to the lithium battery. This technology is notable for its high energy density and low cost per cycle. Helping set them apart from other battery types. However, the term “lithium battery” can be vague as there are around six common chemistries. Each of these batteries has its pros and cons.
Lithium batteries come in two main types: Lithium Metal Batteries and Lithium Ion Batteries (LIBs). Lithium Metal Batteries use lithium in its pure metallic form. Providing a high energy density and making them suitable for applications requiring lightweight and long-lasting power. Some industries that use this type of lithium battery are military and aerospace.

On the other hand, Lithium Ion Batteries utilize lithium compounds and are rechargeable. This makes them ideal for everyday electronics like smartphones, laptops, and electric vehicles. While Lithium Metal Batteries offer greater energy per unit weight, Lithium Ion Batteries are more versatile and safer for repeated use.
Why Lithium battery?
Lithium battery chemistry is favored for its lightweight, quick charging capability, and extended lifespan compared to conventional batteries. They also boast a higher power density, enabling them to offer longer battery life in a more compact and lightweight design.
Due to its position on the periodic table, lithium is one of the lightest metals, located near the top left. However, despite its lightweight nature, lithium is highly reactive and readily gives up electrons, making it a strong reducing agent. This property contributes to its usefulness in batteries.
Lithium Metal Battery
Lithium metal batteries are non-rechargeable batteries with metallic lithium serving as the anode. The name is chosen to differentiate them from lithium-ion batteries, where the cathode is typically made of lithiated metal oxides. Any battery using Li as the negative electrode is classified as lithium
metal battery.
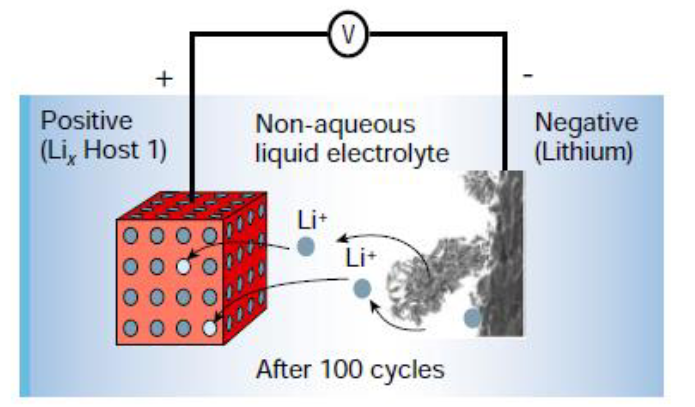
Electrical Cell Potential of a Lithium Metal Battery
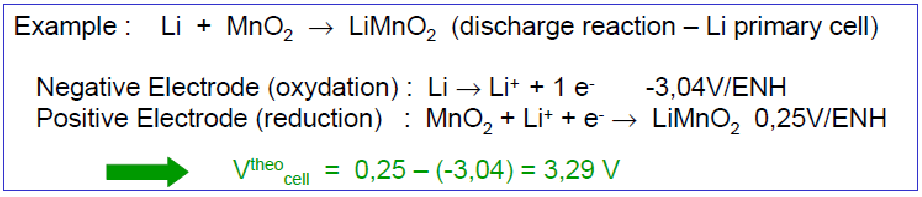
Theoretical Specific Energy of a Lithium Metal Battery

History of Lithium Metal Battery
The history of the Lithium Metal Battery dates back to 1972 when Exxon initiated a significant project. This project utilized TiS2 as the positive electrode, lithium metal as the negative electrode, and lithium perchlorate in dioxolane as the electrolyte. TiS2 was chosen due to its favorable layered-type structure, making it the best intercalation compound available at the time. In the early 1980s, John B. Goodenough proposed the families of compounds known as LixMO2 (where M is Co, Ni, or Mn). All of which are still predominantly used in today’s batteries. These developments marked crucial milestones in the evolution of lithium batteries. Laying the foundation for their widespread use in various applications.
First rechargeable lithium battery
The first rechargeable lithium battery was commercialized by Exxon in 1979, featuring a Li/TiS2 chemistry where lithium reacted with titanium disulfide to form lithium titanium sulfide (LiTiS2). This battery-operated at a cell voltage of approximately 2V and utilized an intercalation reaction at the cathode. However, the commercialization of this battery faced challenges. The lithium anode had poor recharging capabilities due to its highly reactive nature, making it dangerous. Additionally, the combination of a lithium metal anode and a liquid electrolyte led to issues such as uneven lithium growth (dendritic growth) during each discharge-recharge cycle, increasing the risk of explosions. These challenges highlighted the need for safer and more stable lithium battery designs.
How to solve lithium battery safety issues?
The birth of the Li-Ion Battery marked a significant advancement in the safety of rechargeable lithium batteries. To address the safety issues of early lithium batteries, two main approaches were taken.
Firstly, the electrolyte was modified to enhance safety. Traditional liquid electrolytes were replaced with polymer electrolytes or solid-state electrolytes, which are less prone to leakage and dendritic growth, reducing the risk of explosions.
Secondly, modifications were made to the negative electrode. Instead of using metallic lithium, which was prone to dendritic growth and instability, a second insertion material, such as graphite, was used. This change allowed for a safer and more stable battery operation.
These modifications, along with ongoing research and development, have significantly improved the safety and performance of rechargeable lithium batteries, making them a vital power source for numerous applications.
Li-Ion Battery
Lithium-ion is currently the most widely used rechargeable battery chemistry. These batteries are responsible for powering many of our daily devices, such as smartphones and electric vehicles. Lithium-ion batteries are a type of rechargeable cells that utilize lithium intercalation reactions in both electrodes, with lithium ions moving between them in a “rocking chair” framework. However, it took nearly a decade to realize the Li-ion concept due to delays caused by the absence of suitable materials for the negative electrode.
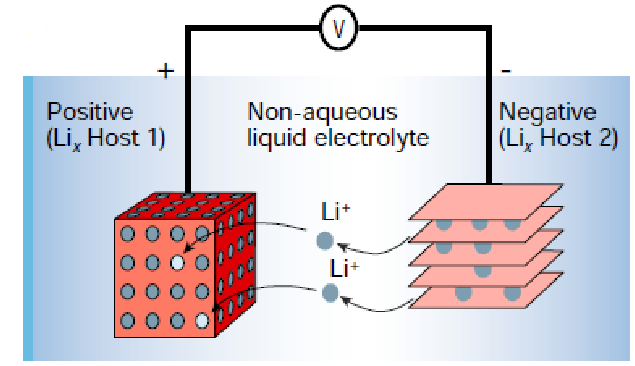
Li-ion batteries store energy by reversibly intercalating Li+ ions into electronically conducting solids. Compared to other commercial rechargeable batteries, they are known for their higher specific energy, energy density, energy efficiency, longer cycle life, and calendar life. An impressive advancement was seen in Li-ion battery properties after their market introduction in 1991: over the next 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.
Types of Li-Ion Battery
There are several types of Li-ion batteries available, distinguished by the composition of their cathode (positive electrode). These include:
- LiCoO2: Standard lithium-cobalt-oxide battery, known for its high energy density but with limited thermal stability and a tendency for capacity degradation over time.
- LiMnNiCoO2: Lithium-manganese-nickel-cobalt-oxide battery, offering a balance between energy density, power capability, and thermal stability, commonly used in electric vehicles.
- LiFePO4: Lithium-iron-phosphate battery, known for its excellent safety, long cycle life, and thermal stability, is often used in applications where safety is critical.
- LiMnO2: Lithium-manganese-oxide battery, offering high power capability but with lower energy density compared to other Li-ion chemistries.
- LiNiO2: Lithium-nickel-oxide battery, known for its high specific energy and thermal stability, is commonly used in high-performance applications.
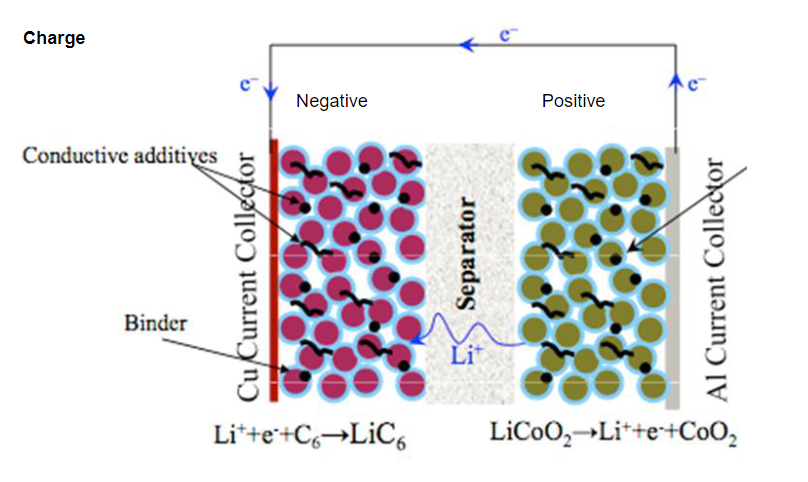
Li-ion Battery Electrode Potentials
Li-ion battery electrode potentials exhibit a large voltage difference between the anode and cathode, which is crucial for generating electrical energy. This potential, typically around 3-4 volts, is essential for the battery’s operation. However, this voltage difference mustn’t exceed the electrolyte’s stability window. If the voltage exceeds this limit, it can lead to the breakdown of the electrolyte, causing irreversible damage to the battery and potentially compromising its safety and performance. Therefore, managing and controlling the electrode potentials within the electrolyte stability window is critical for ensuring the efficiency and longevity of Li-ion batteries.
History of the Lithium-ion Battery
The history of the Lithium-ion Battery begins with many attempts in the 1960s and 1970s to create batteries using lithium metal anodes and conversion cathodes, such as Li/CuCl2. These batteries had a theoretical cell voltage of 3.07V but struggled to deliver many charge/discharge cycles. In 1979, Exxon commercialized the first rechargeable lithium battery, using a Li/TiS2 chemistry with a cell voltage of around 2V, which utilized an intercalation reaction at the cathode.

However, the lithium anode recharged poorly. The breakthrough came in 1991 when Sony commercialized the first rechargeable lithium-ion battery, using a LixC + LiyCoO2 chemistry with a cell voltage of approximately 3.7V. This battery employed intercalation reactions at both electrodes, marking a significant advancement in battery technology.
Li-ion Battery Applications
Li-ion batteries find wide applications in various industries. Cell phones and smartphones constitute the largest single market, with wound prismatic cells being dominant, primarily using LiCoO2 chemistry. In the laptop and tablet computer market, Li-ion batteries are rapidly replacing traditional PCs, with LiCoO2 being phased out in favor of mixed metal oxide. In power tools, high-power cylindrical cells are prevalent, although LiFePO4 is being replaced by mixed metal oxide.
Electric and hybrid vehicles represent a significant market for Li-ion batteries, with projections indicating it could be the largest. However, challenges such as cost, lifetime, safety, and low-temperature performance hinder widespread adoption. The existing global market for Li-ion batteries is approximately $58.21 billion, with projections suggesting it could exceed $293 billion by 2032.
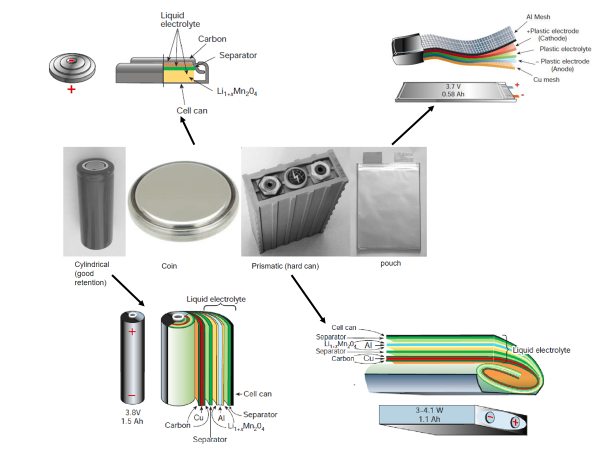
Li-Ion Cell Design
Lithium-ion cell design refers to the physical format or shape of the cell. Li-ion cells are available in four basic formats:
- Cylindrical: These cells are shaped like traditional AA batteries and are commonly used in laptops and power tools. They are known for their durability and high energy density.
- Coin: Coin cells are flat, round cells often used in small electronic devices like watches, calculators, and some medical devices. They are compact and lightweight.
- Pouch: Pouch cells are flexible, flat cells that can be manufactured in various sizes and shapes. They are commonly used in smartphones, tablets, and other portable electronics due to their thin profile and lightweight design.
- Prismatic: Prismatic cells are rectangular and are often used in larger devices where space is not a constraint, such as electric vehicles and energy storage systems. They offer good energy density and are more cost-effective to manufacture than cylindrical cells.
What are dendrites in a Lithium Battery?
Dendrites in a battery are branch-like projections of metal that can form on the surface of lithium. These dendrites pose a significant safety risk in lithium-ion batteries because they can grow to pierce through the separator, creating an electrical short circuit between the anode and cathode. This can lead to catastrophic failure of the battery.
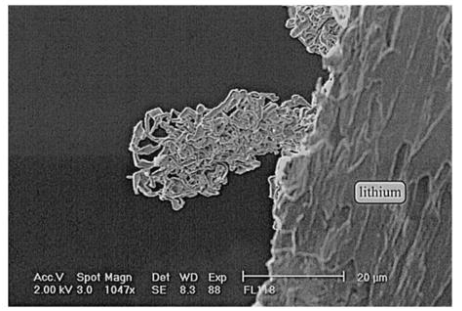
Formation of lithium dendrites on the lithium metal foils
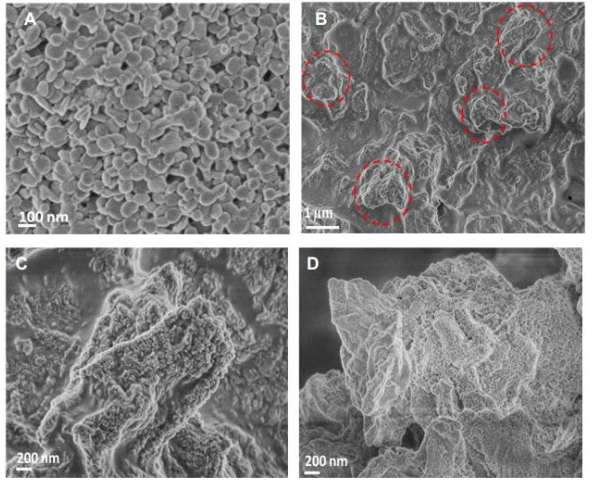
A) SEM image from a top view of a standard lithium metal foil after just a few tens of charge/discharge cycles, displaying the development of spherical, globule-like dendritic projections.
B) With continued cycling, these globules merge to form larger micron-scale projections. This SEM image depicts a typical lithium foil after numerous charge/discharge cycles, showing several dendritic projections ranging from a few hundred nanometers to tens of microns in size.
C) Close-up SEM image of a typical dendritic projection extending from the lithium metal foil.
D) Close-up image of the tip region of a sharp dendritic projection. It is worth noting that these projections are oriented perpendicular to the lithium foil and point toward the separator.
All samples (A-D) were examined following a full insertion of lithium into the lithium metal foil.
How does dendrite form?
Dendrites form in the case of bulk metallic lithium, such as foils, due to non-uniformities in the structural morphology. This causes a non-uniform distribution of current, leading to localized excessive charge densities. As a result, lithium metal is unevenly plated, leading to the formation of dendritic projections. Recent studies have shown that these structural non-uniformities originate not on the surface, as previously believed, but in the sub-surface of the lithium electrode. Over time, these sub-surface dendrites grow and eventually burst out of the surface, protruding into the electrolytes.
What happens if a lithium-ion battery is overcharged?
When a lithium-ion battery is overcharged, the chemical reaction at the cathode (LiCoO2) results in the generation of lithium ions (Li+), cobalt dioxide (CoO2), and an electron (e–). This reaction is irreversible, and continuous overcharging ages the Li-ion battery quickly. Overcharging can lead to the battery overheating, exploding, and causing fires. It’s important to note that the explosion is not caused by gas, but rather by the buildup of internal pressure due to overheating. Even a slight overcharge can reduce a cell’s discharge capacity, leading to overcharging, increased impedance, heat generation, and ultimately, a decrease in cell lifetime.
Why are typical Li-ion cells only charged to ~4.2V?
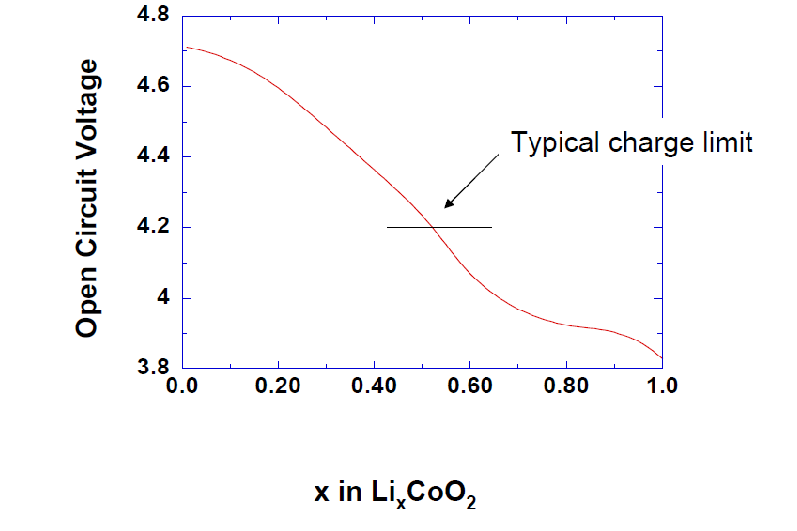
Typical Li-ion cells are only charged to around 4.2V due to several factors. Firstly, standard commercial Li-ion electrolytes are only stable up to approximately 4.5V versus Li/Li+. Charging beyond this voltage could lead to electrolyte instability, potentially resulting in cell failure or safety hazards. Additionally, the thermodynamic stability of the cathode material, such as LixCoO2, is also a concern. When x (the amount of lithium in LixCoO2) is between 0.5 and 1.0, the compound is stable. However, if x is less than 0.5, LixCoO2 can decompose into Li2O, Co2O3, Co3O4, or O2, which can degrade the cell and reduce its performance. Therefore, to ensure the safety and longevity of Li-ion cells, they are typically charged only to around 4.2V.
Lithium Battery Safety
It is crucial to prioritize Li-ion battery safety due to the potential for catastrophic failures. The failure rate in commercial Li-ion batteries is estimated to be around 1 in 5-10 million, which may seem low but translates to approximately 1 in 1 million laptops and 1 in 1000 Tesla Roadsters. These statistics underscore the importance of implementing robust safety measures, such as a good battery management system, to mitigate the risks associated with Li-ion batteries. Such systems help monitor and control the battery’s operation, ensuring safe charging and discharging to prevent overheating, short circuits, and other hazardous conditions.

Conclusion
In conclusion, lithium batteries have revolutionized the way we power our portable electronics, electric vehicles, and even homes. Their high energy density, long cycle life, and relatively low cost per cycle make them an attractive choice for a wide range of applications. However, challenges such as dendrite formation, electrolyte instability, and safety concerns during charging and discharging remain areas of active research. As technology continues to advance, lithium batteries are expected to play an even larger role in our lives, powering the devices and vehicles of the future.