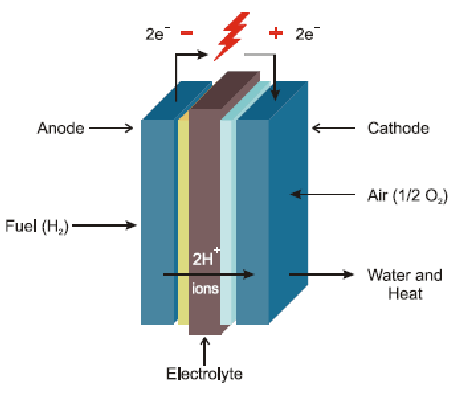
Basics of Electrochemistry
Electrochemistry is the exploration of chemical reactions that induce the motion of electrons, which is called electricity. It is a highly interdisciplinary field with applications spanning physical, chemical, and biological sciences. Today, we are going to explore various areas where electrochemistry plays a crucial role. These include energy conversion and storage, focusing on fuel cells and lithium-ion batteries.
What is electrochemistry in simple words?
Electrochemistry is a branch of chemistry that studies the relationship between electricity and chemical reactions. It involves the study of electron flow during oxidation or reduction reactions.
How does electrochemistry generate electricity?
Atoms generate electricity through the movement of electrons from one element to another. Each element undergoes oxidation or reduction at a distinct electrical potential. This reaction is known as a redox (oxidation-reduction) reaction. In redox reactions, electrons are transferred between different elements, creating an electric current.
Why should I care about electrochemistry?
Electrochemistry is crucial in various fields, including energy storage, corrosion prevention, and electroplating. In electrochemistry, several primary variables are essential for understanding and analyzing electrochemical processes. These primary variables include current, voltage, and charge. Each of these variables plays a distinct role in the behavior of electrochemical systems.
What are examples of electrochemistry?
Examples of electrochemistry include fuel cells, which convert the chemical potential energy from the oxidation of fuels like hydrogen gas, hydrocarbons, or alcohols into electrical energy. Other examples include torches and electronics such as cellphones, powered by long-life alkaline batteries, digital cameras using lithium-ion batteries, and hearing aids powered by silver-oxide batteries.
How to calculate current in electrochemistry?
Current, measured in amperes (A), is a fundamental variable in electrochemistry. It represents the flow rate of electric charge in a circuit. Current is defined as the rate of flow of coulombs or electrons per second. One ampere is equivalent to one coulomb per second or approximately 6.24 × 1018 electrons per second. Current is a crucial parameter in electrochemical systems as it determines the electrochemical reaction rate at the electrodes.
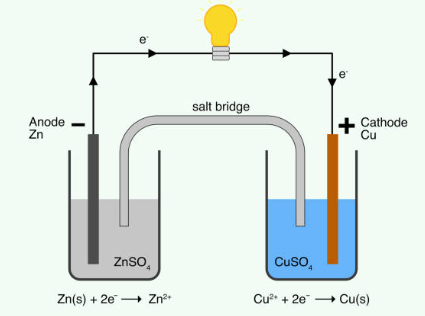
What does voltage mean in Electrochemical cells?
Voltage, measured in volts (V), is another essential variable in electrochemistry. The presence of voltage in an electrochemical cell signals that the system is not in equilibrium. It represents the energy available to drive electric charge externally between the electrodes of an electrochemical cell. One volt is defined as one joule per coulomb or, equivalently, one joule per 6.24 × 1018 electrons. Voltage provides valuable information about the thermodynamics of electrochemical processes. It is also essential to understand the driving force behind these reactions.
What is charge transfer in electrochemistry?
Charge, measured in coulombs (C), is a fundamental quantity in electrochemistry. Charge transfer refers to the movement of electrons from one atom or molecule to another. This occurs when one entity has an excess of free electrons or a propensity to lose electrons, and the other entity has an attraction for those electrons. One coulomb is equivalent to 6.24 × 1018 electrons. Charge is crucial for quantifying the extent of electrochemical reactions. It is often used to calculate the amount of material transformed during these processes.
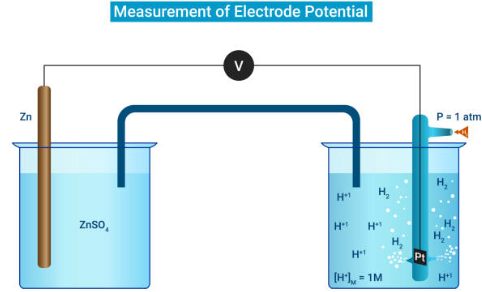
Additionally, the concept of a mole of electrons is used in electrochemistry. One mole of electrons is equivalent to 6.02 × 10^23 electrons, or one Faraday (F), which is approximately 96485.4 coulombs. One Faraday of charge corresponds to one ampere of current flowing for 96485.4 seconds, which is approximately 26.8 hours.
Faraday’s laws of electrolysis
The laws of electrochemistry dictate that the extent of chemical change at an electrode-electrolyte interface is directly proportional to the amount of electricity passed through the system, and the quantity of chemical changes resulting from the same amount of electricity in different substances is directly proportional to their respective equivalent weights.

Faraday’s law is fundamental in understanding the relationship between electric charge and chemical reactions in electrochemistry. It states that the passage of 96,485.4 coulombs of charge (which is equivalent to one Faraday) causes one equivalent of a chemical reaction to occur. An equivalent of a reaction is defined as the consumption of one mole of reactant or the production of one mole of product in a one-electron reaction.
As a result, this law highlights the stoichiometric relationship between the number of electrons that cross an interface in an electrochemical cell. The extent of the chemical reaction, specifically the amounts of reactant consumed and product generated. Faraday’s law is essential for quantifying electrochemical processes and is used extensively in the design and analysis of electrochemical systems.
Electrosynthesis
Electrosynthesis involves using electrical energy to create a material, often associated with organic chemistry for making chemical compounds in solution. This process uses anodic and cathodic reactions to generate reactive intermediates at the electrode surface, which then react in the solution to form the desired product. Examples include the Kolbe reaction and the production of adiponitrile for nylon 66.
Electroplating, another common application, reduces a metal salt in solution to its metallic form at the cathode, used in various industries including semiconductors and jewelry. Electrodeposition, the process of depositing material at an electrode, can create diverse materials like nanostructured metals and polymers, with properties controlled by factors such as electrolyte solvent, temperature, and applied potential.
What is an example of electrosynthesis?
An example of electrosynthesis is the production of medicinal agents like analgesics and disinfectants, as well as synthetic rubber materials such as nitrile. Electrosynthesis involves using an electric current to drive targeted chemical reactions, creating specific intermediate or end products.
Corrosion
Corrosion refers to the deterioration of metals caused by electrochemical reactions, where the metal oxidizes to form an oxide or metal salt upon contact with an oxidant in water or gas. This process involves the flow of electrons from the metal (anode) to the oxidant (cathode) through differences in electrical potential, creating an electrochemical cell.

Factors affecting corrosion include the kinetics and thermodynamics of the reactions, as well as the size of the anodic and cathodic regions. Other factors like pH, surface defects, and the formation of insulating corrosion products or porous deposits also influence corrosion rates. Corrosion is not limited to metals and also includes the degradation of polymers and ceramics.
What is the electrochemical principle of corrosion?
The electrochemical principle of corrosion involves the transfer of electrons from the surface of a metal to a suitable electron acceptor or depolarizer. This process requires the presence of water, which serves as a medium for ion transport. Common depolarizers include oxygen, acids, and cations of less active metals.
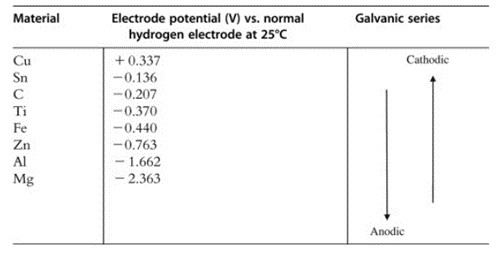
How can we prevent galvanic corrosion between steel and aluminum?
Whenever dissimilar materials come in contact, they always have the potential for galvanic corrosion to occur. When an electrolyte, such as water, encounters components that have a difference in electrode potential, the material with the lower electrode potential acts as the anode, and the other material with a greater electrode potential acts as the cathode, creating a corrosion battery.
The anode dissolves in the electrolyte and the corrosion particles accumulate on the cathode. Galvanic corrosion between dissimilar materials can be avoided or diminished by inserting electrical insulation between the two materials or by applying a coating such as hot dipping galvanized on one or all materials.
What is the hot dip galvanizing process?
The process of hot dip galvanization is when a clean piece of steel is immersed in a bath of molten zinc to obtain a coating that will protect the steel from corrosion. Carbon steel can also be coated with an aluminum-silicon alloy, this aluminum-coated steel is known as aluminized steel.
How is electrochemistry used in everyday life?
Electrochemistry finds numerous everyday applications. Batteries, ranging from those in flashlights to calculators and automobiles, use chemical reactions to produce electricity. Additionally, electricity is employed in electroplating processes to coat objects with decorative metals such as gold or chromium.
Conclusion
In conclusion, understanding the basics of electrochemistry is crucial for grasping the principles behind many chemical and biological processes. This field not only provides insights into how batteries and fuel cells work but also plays a vital role in industries such as metal plating and corrosion prevention. By delving into concepts like current, voltage, and charge, we uncover the intricate mechanisms of electron transfer and chemical reactions at the heart of electrochemistry. As we continue to explore and apply these fundamentals, we unlock new possibilities for sustainable energy, innovative materials, and advancements in various scientific disciplines. Electrochemistry truly stands as a cornerstone of modern science, paving the way for exciting discoveries and technological breakthroughs.
Table Summary
| Conversion | Value |
|---|---|
| 1A = 1 coulomb/second | 6.24 × 1018 electrons/second |
| 1V = 1 joule/coulomb | 1 joule/6.24 × 1018 electrons |
| 1 coulomb (C) | 6.24 × 1018 electrons |
| 1 mole of electrons | 6.02 × 1023 electrons |
| 1 mole of electrons = 1 Faraday (F) | 96485.4 C |
| 1 Faraday (F) | 1A for 96485.4 seconds (26.8 hrs) |