Electric Battery Explained
An electric battery is an energy storage device comprising one or more electrochemical cells. These cells have external connections used to power electrical devices. When providing power, the battery’s positive terminal serves as the cathode, while the negative terminal functions as the anode. Electrons flow through an external electric circuit to the positive terminal from the negative terminal.

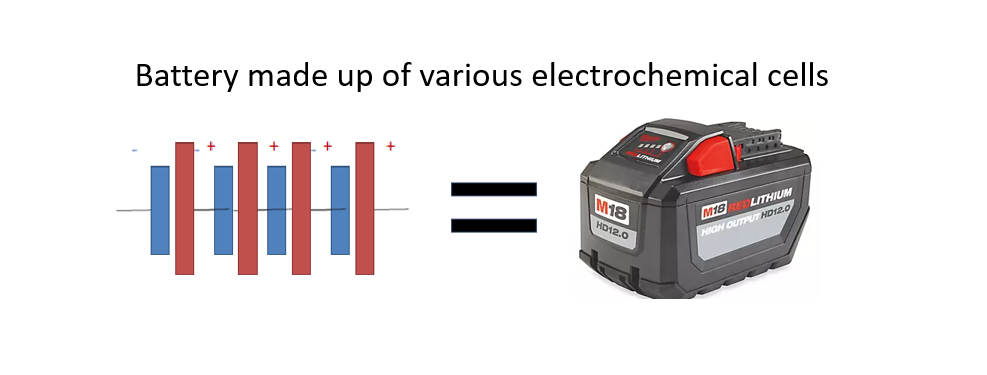
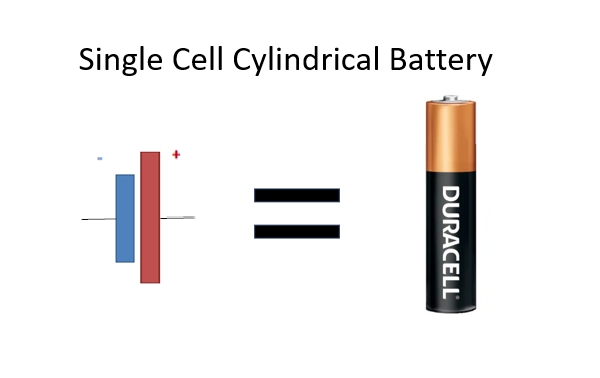
When connected to an external load, a redox reaction within the battery converts high-energy reactants into lower-energy products. This releases the energy difference to the external circuit as electrical energy. Initially, “battery” referred to a device of multiple cells. However. its usage has expanded to include single cell’s think of a single cell AA /AAA battery.
What is an Electric Battery?
A battery is a mechanism designed to store chemical energy and convert it into electrical energy through a process known as electrochemistry. The fundamental unit of a battery is an electrochemical cell, which comprises two electrodes separated by an electrolyte. A battery can consist of one or multiple electrochemical cells, as seen in Volta’s original pile.
A battery is usually comprised of several electric cells. These cells produce a direct current through the conversion of chemical energy into electrical energy
What is a battery cell?
A battery cell combines two electrodes arranged so that an overall oxidation-reduction reaction produces an electromotive force.
What is the role of electrolytes in an electric battery?
Electrolytes play a crucial role in a battery by facilitating the movement of ions between the two electrodes. In a lithium-ion battery, the electrolyte is a liquid that allows lithium ions (Li+) to move between the anode and cathode during charging and discharging. This movement of ions is essential for the battery to function, enabling electric current flow.

The electrolyte also serves to stabilize the surfaces of the cathode and anode, which helps extend the lifespan of the battery. Additionally, lithium-ion batteries’ electrolyte chemistry is well-defined, contributing to their reliability and efficiency. Overall, the electrolyte is a pivotal battery component, playing a critical role in its performance and longevity.
What electrolytes are used in batteries?
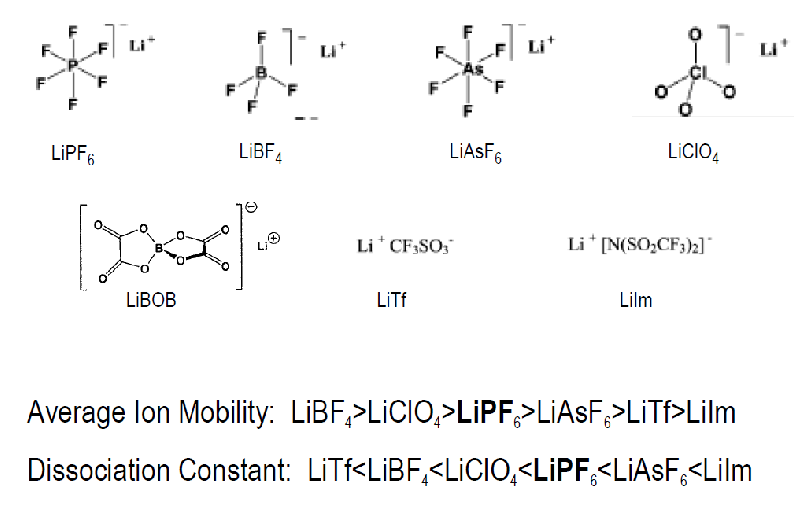
In household alkaline batteries, the electrolyte is potassium hydroxide. In lithium batteries, the most typical electrolyte is a solution of lithium salt, such as lithium hexafluorophosphate (LiPF6).
What are the desired Properties for a Electric Battery Electrolyte?
The desired properties for an electric battery electrolyte include acting as a medium for ion transport, comprising solvent(s), salt(s), and additive(s). It should have a high concentration of dissolved electrolyte salt and a high dielectric constant solvent to facilitate high ionic conductivity. Low viscosity is also essential for enabling high ionic mobility and, consequently, high conductivity. Additionally, the solvent should be non-reactive with the electrodes. Characteristic properties of an electrolyte include its electrical conductivity, measured in Siemens/cm or ohm-1cm-1, and its voltage window, which determines the anodic and cathodic voltage limits where the electrolyte is oxidized or reduced.
Electric Battery Time Line
The timeline of battery development spans centuries, beginning with intriguing discoveries like the terracotta jars found in Baghdad around 100 B.C.E., which some believe may have been the first battery. In 1789, Alessandro Volta created the first modern battery using zinc, an electrolyte, and copper. The 19th century saw significant advancements, with Gaston Planté inventing the first rechargeable lead-acid battery, in 1859.
Subsequent innovations included the first zinc/carbon “dry cell” in 1887 and the first nickel-cadmium (Ni/Cd) rechargeable battery in 1899. The 20th century brought more milestones, such as the invention of the alkaline battery by Eveready in 1955 and the development of the nickel-metal hydride (Ni/MH) battery in the 1980s to replace Ni/Cd due to environmental concerns. One of the most significant advancements came in 1991 when Sony introduced lithium-ion cells, revolutionizing portable electronics with their high energy density and rechargeable capabilities.
How is chemistry involved in batteries?
Imagine you have a battery comprised of two materials [A] and [B]. Material A is prone to giving up electrons and Material B is prone to taking them. If this battery does not have an electrolyte separating both elements, both elements will react with one another until all that is left inside this battery is AB. Based on the thermodynamic principle of Gibbs free energy both elements A and B would combine to create AB. The byproduct of this chemical reaction would be heat (wasted energy).
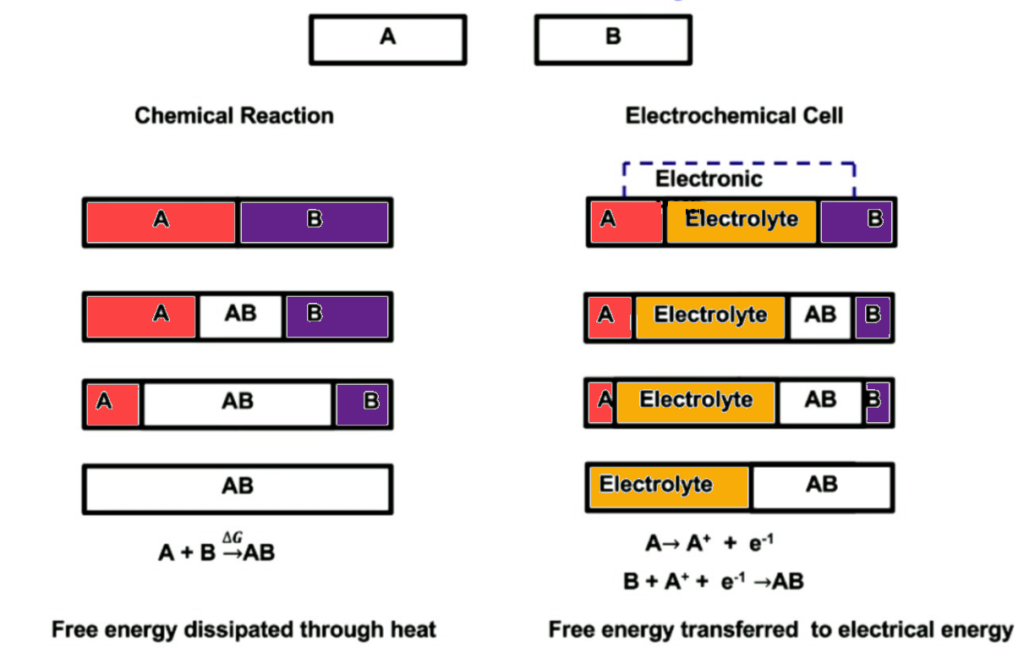
So now what happens when electrolyte is present in between material A and B? So the electrolyte is a chemical substance that will allow A+ ions to pass through it. That way material A can still react with material B. It’s important to remember that material A is prone to giving up electrons, so it has no problem leaving its electrons behind. However, the electrolyte does not allow for the electrons to travel through it. As a result, you are left with “free” electrons on side A.
If we then take an electrically conductive material such as copper we connect sides A and B. The Electrons will naturally travel from A to B. These electrons that are traveling from A to B can then be put to work but add a load to this newly formed electrical circuit.
During the flow of electricity, the cathode gains an equal number of electrons to those lost by the anode. This process occurs through two distinct chemical reactions. The reduction, where the cathode gains electrons, and oxidation, where the anode loses electrons.
What is Oxidation/Reduction?
Oxidation and reduction are key concepts in chemistry, often referred to as redox reactions. Oxidation is a reaction in which an atom or molecule loses one or more electrons. Conversely, reduction is a reaction in which an atom or molecule gains one or more electrons. These two processes often occur together in what is known as a redox reaction, where the number of electrons gained is equal to the number of electrons lost. In simple terms, oxidation is the loss of electrons, and reduction is the gain of electrons.
Electric Battery Construction
Electric battery construction involves several key components that work together to store and deliver electrical energy.
- Anode (Negative Electrode): The anode is where the oxidation reaction occurs during discharge, releasing electrons into the external circuit. Common anode materials include graphite and lithium compounds in lithium-ion batteries.
- Cathode (Positive Electrode): The cathode is where the reduction reaction occurs during discharge, accepting electrons from the external circuit. Cathode materials vary widely depending on the battery type, such as lithium cobalt oxide (LiCoO2) in lithium-ion batteries.
- Separator: The separator is an insulating material placed between the anode and cathode to prevent electrical short circuits while allowing the flow of ions between the electrodes. It is typically made of a porous material that can absorb and hold the electrolyte.
- Current Collector: The current collector is a conductive material that collects and delivers electrons to and from the external circuit. It is usually made of metal foil, such as copper or aluminum, and is coated with the active electrode material.
- Electrolyte: The electrolyte is a chemical substance that provides the ions necessary to support the electrochemical reactions at the electrodes. Depending on the battery type, it can be liquid, gel, or solid. Common electrolytes include lithium salts in a solvent for lithium-ion batteries.
- Enclosure Hardware: The enclosure hardware includes the casing and terminals that hold the battery components together and provide connections for external devices. The casing is often made of metal or plastic, while the terminals are typically metal contacts for conducting electricity in and out of the battery.
Form Factor of an Electric Battery
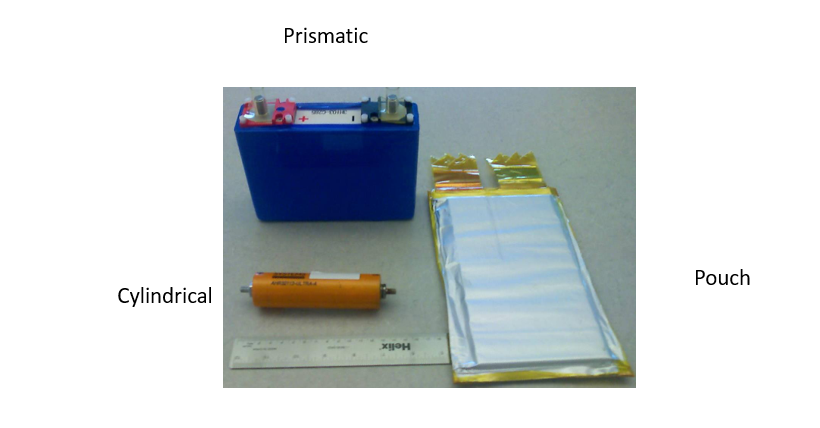
Alright, imagine an electric battery as the power-packed heart of your favorite device or vehicle. When we talk about its form factor, we’re describing its physical shape and structure. There are 3 main types: prismatic, pouch, and cylindrical. The prismatic battery is like a neat stack of plates, with no round edges. Its positive and negative plates are stacked flat, unlike the cylindrical cell where they’re rolled up. Within the prismatic category, there are even more variants, like the pouch and the hard can, each with its unique design and characteristics. These different form factors offer manufacturers flexibility in designing batteries to fit various devices and applications.
The term “battery” is often used colloquially to refer to a single battery cell, but some purists argue that it should only be used to describe a device composed of multiple cells. A battery cell consists of two half-cells, each producing a voltage. When multiple cells are wired together in series and/or parallel configurations, they form a battery module.
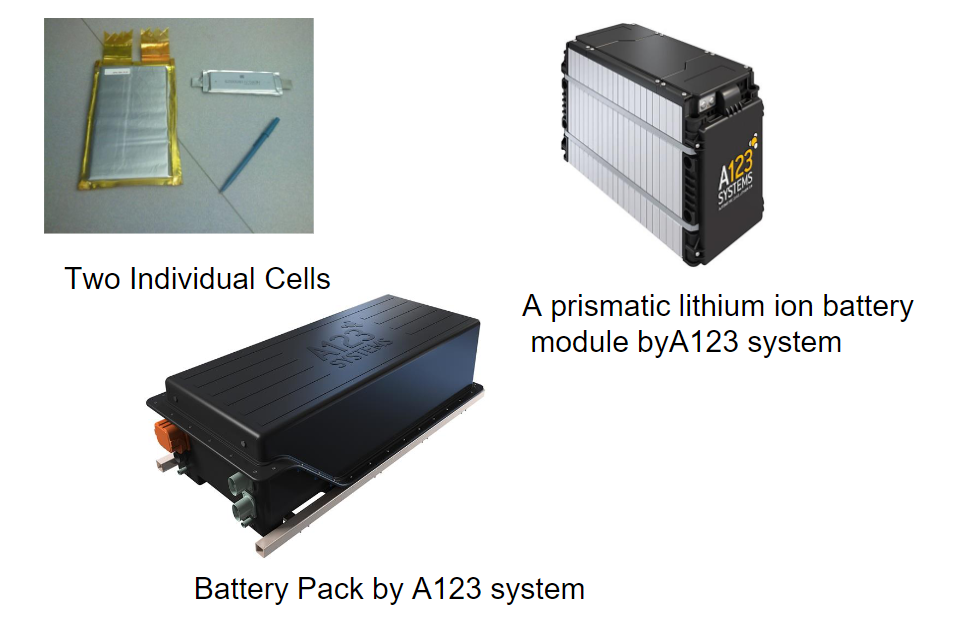
Several of these modules can then be combined to create a battery pack, which is the final power source used in various applications. This hierarchical structure demonstrates the complexity and versatility of modern battery systems, which can range from simple single cells to intricate packs powering electric vehicles and complex electronic devices.
Electric Battery Parameters
| Term | Definition | Units | Equations |
|---|---|---|---|
| OCV (V) | Open Circuit Voltage – Cell voltage with no current running through the cell. | Volts | N/A |
| Watt-hour (Wh) | The power capability of a battery per unit volume. | Watt-hours | N/A |
| Cell Capacity | The total amount of electrical charge that a cell can hold. | Amp-hours (Ah) | Energy capacity (watt-hours) = Ah * cell voltage |
| Cell Voltage | The potential difference between the anode and the cathode. | Volts | N/A |
| Energy Density | The total amount of electrical energy stored by a battery per unit of volume. | Watt-hours/liter (Wh/L) | N/A |
| Power Density | The power capability of a battery per unit weight. | Watts/liter (W/L) | N/A |
| Specific Energy | The total amount of electrical energy stored by a battery per unit of weight. | Watt-hours/kilogram (Wh/kg) | N/A |
| Specific Power | The charge or discharge rate is a function of the cell’s capacity – a normalized discharge/charge rate, or current. | Watts/kilogram (W/kg) | N/A |
| C-Rate (A) | The charge or discharge rate as a function of the cell’s capacity – a normalized discharge/charge rate, or current. | Amperes (A) | FoE.g. For a cell with 1 Ah of capacity, a 1C discharge would be 1 A, a 2C discharge would be 2 A, and a C/2 discharge would be 0.5 A. |
| State of Charge (SOC) | The amount of capacity available to discharge relative to the theoretical capacity – A measure of how far a device is discharged. | Percentage (%) | N/A |
| Depth of Discharge | Capacity consumed relative to theoretical capacity – 1-SOC; Also a measure of how far a device is discharged. | Percentage (%) | N/A |
| Cycle Life | Accumulated number of charge/discharge cycles before battery capacity falls below a given percentage of its original capacity. | Number of cycles | Example: “300 cycles to 80% of original capacity” |
| Self Discharge | The rate at which a battery loses capacity on standing. | Percentage per unit time (%) | N/A |
Cell Connections – How They Affect Capacity and Voltage
Cell connections play a crucial role in determining the capacity and voltage of an electrical system. When cells are connected in series, the voltages of the individual cells add up. For example, if you connect two 1.5V cells in series, the total voltage would be 3V. This increase in voltage is beneficial for applications that require higher voltages.
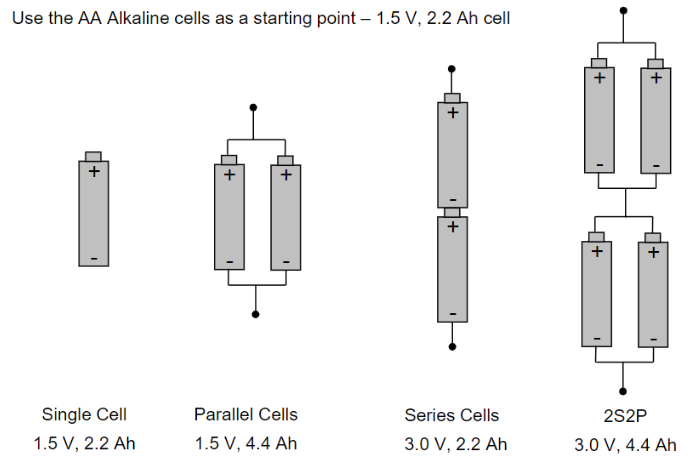
On the other hand, connecting cells in parallel increases the overall capacity of the system, measured in amp-hours (Ah). For instance, if you connect two 1Ah cells in parallel, the total capacity would be 2Ah. This configuration is useful for applications that require longer runtime or higher current output.
Combining series and parallel connections can increase both the voltage and the capacity of the system. For instance, connecting four sets of two cells in series and then connecting these sets in parallel would result in a system with four times the voltage of a single cell and twice the capacity.
In summary, series connections increase voltage, parallel connections increase Ah-capacity, and combinations of both can increase both voltage and Ah-capacity in an electrical system.
Common types of batteries
Below are a few common types of electric battery/ energy storage systems.
- Lithium-Ion Batteries: These are commonly used in portable consumer electronics due to their high energy density, power-to-weight ratio, efficiency, and long life. They are also used in most all-electric vehicles and PHEVs, with ongoing research to reduce costs and improve safety.
- Nickel-Metal Hydride Batteries: These offer reasonable energy and power capabilities, with a longer life cycle than lead-acid batteries. They are commonly used in HEVs but face challenges such as high cost, self-discharge rate, and heat generation.
- Lead-Acid Batteries: These are inexpensive, safe, and reliable but have low energy density, poor cold-temperature performance, and short life cycles. They are primarily used for ancillary loads in electric-drive vehicles and stop-start functionality in internal combustion engine vehicles.
- Ultracapacitors: While they have low energy density, ultracapacitors offer very high power density, making them useful for delivering high power quickly during acceleration and hill climbing. They can also help recover braking energy and level load power in electric-drive vehicles.
Battery System Engineering
Battery System Engineering is an interdisciplinary field that involves the collaboration of various specialists to design, develop, and optimize battery systems. Chemists and material scientists play a crucial role in understanding the chemical processes within the battery and developing new materials to improve performance. Dynamic modelers use mathematical models to simulate the behavior of the battery system under different conditions, helping to predict its performance and optimize its design.
System engineers are responsible for integrating the battery into larger systems, ensuring that it meets the overall requirements and functions correctly within the system. Electrical engineers focus on the electrical aspects of the battery system, such as designing the electrical circuits and ensuring proper voltage and current management. Mechanical engineers, on the other hand, are involved in the physical design of the battery system, including packaging, thermal management, and mechanical integration.
Overall, Battery System Engineering brings together experts from various disciplines to create efficient, reliable, and safe battery systems for a wide range of applications, from consumer electronics to electric vehicles and renewable energy storage.
Conclusion
In conclusion, electric batteries are fascinating devices that play a crucial role in our daily lives, powering everything from our smartphones to electric vehicles. Understanding the basic principles of how batteries work, such as the electrochemical processes involved and the different types of batteries available, can help us make informed decisions about their use and care. As technology advances, we can expect to see further improvements in battery technology, leading to more efficient and sustainable energy storage solutions for the future.