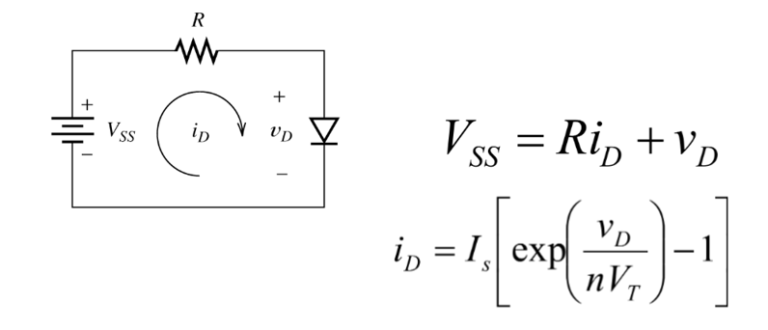What is a Fuel Cell?
A fuel cell is a device that converts chemical energy directly into electrical energy, water, and heat through electrochemical reactions. It consists of an anode and a cathode separated by an electrolyte, which is typically a porous membrane. Hydrogen, and air (or oxygen) are fed into the cell. At the anode, the fuel undergoes a reaction that releases electrons and generates ions. The electrons flow through an external circuit, creating an electric current. Meanwhile, the ions travel through the electrolyte to the cathode. At the cathode, the electrons and ions combine with oxygen from the air to produce water and heat.
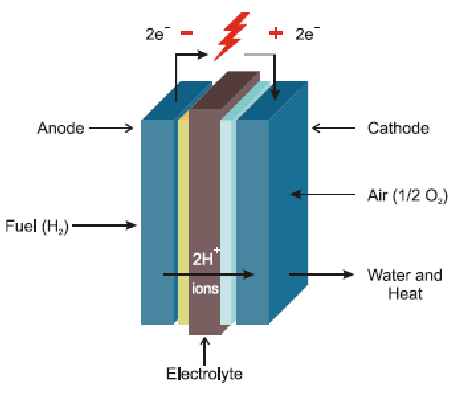
This process is efficient and produces few emissions, making fuel cells a promising technology for clean energy production. Although the voltage generated by a single cell is typically low (< 1 volt). By connecting multiple cells in series we can produce a higher voltage, making them suitable for various applications. Some common applications of fuel cells include hydrogen cars and providing backup power.
History of the Fuel Cell
The history of fuel cells dates back to 1839 when William Grove first discovered the principle. He used four large cells, each containing hydrogen and oxygen, to produce electric power. However, serious interest in this technology as a practical generator did not begin until the 1960s.
NASA demonstrated the commercial potential of fuel cells in the 1960s on the Gemini and Apollo space flights, although these early fuel cells were very expensive. Since the 1970s, fuel cell research and development have been actively pursued in commercial applications for fuel cells. These range from low-cost portable systems for cell phones and laptops to large power systems for buildings.
Why don’t we use fuel cells?
Simply put raw material cost. Fuel cells and certain water electrolyzers often necessitate the use of valuable metals like platinum and iridium as catalysts. As a result, the initial investment in fuel cells and electrolyzers can be substantial. This elevated cost has dissuaded some individuals from considering / further developing hydrogen fuel cell technology.
Understanding Fuel Cell Technology
Fuel cell technology operates on the principle of electrochemical reactions. In a fuel cell, the fuel is oxidized at the anode (negative electrode), and the oxidant is reduced at the cathode (positive electrode). Ions are transported between the electrodes through an electrolyte, while electrons flow through an external circuit, generating an electrical current.
How Fuel Cells Work:
In a hydrogen fuel cell, the fuel gas (which is hydrogen-rich) is supplied to the anode. At the anode, hydrogen molecules (H2) undergo oxidation, releasing protons (H+) and electrons (e–):
H2 (g) = 2H+ + 2e-
The electrons produced at the anode cannot pass through the electrolyte, so they flow through the external circuit, where they perform work, such as powering a device, before reaching the cathode. Meanwhile, the positive hydrogen ions (H+) migrate across the electrolyte to the cathode. At the cathode, these ions combine with oxygen (from the air) and electrons from the external circuit to form water as a byproduct. Overall, the fuel cell reaction can be summarized as:
2H+ + 1/2 O2 + 2e– = H2O + Heat
fuel + oxidant = product + Heat
Fuel cells are efficient and produce electricity without combustion, making them a clean and promising technology for various applications, including transportation and stationary power generation.
What is Electrolysis and What does this have to do with fuel cells?
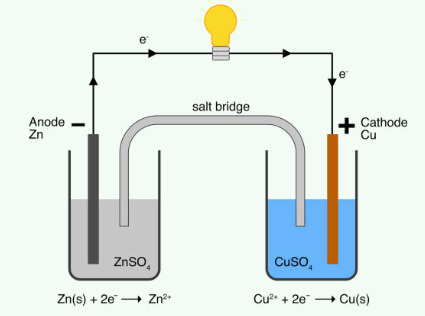
Electrolysis is a process that uses electricity to split water (H2O) into its constituent elements, hydrogen (H2) and oxygen (O2). This is achieved by passing an electric current through water, which causes the water molecules to break apart. The oxygen is typically collected at the anode, while the hydrogen is collected at the cathode. The connection between electrolysis and fuel cells lies in their ability to reversibly convert between electrical energy and chemical energy. In electrolysis, electrical energy is used to produce hydrogen and oxygen from water.
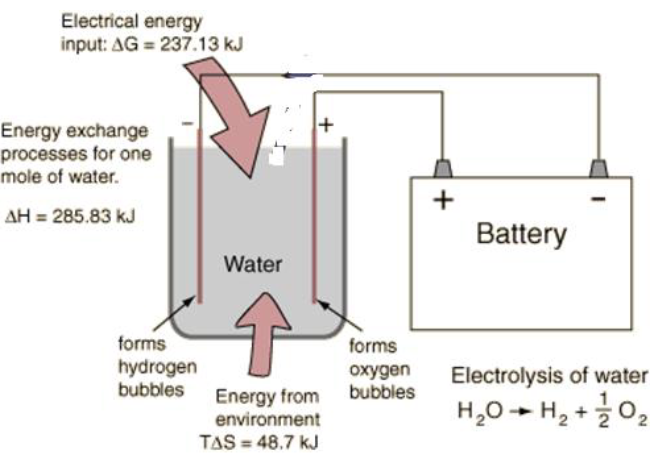
In a fuel cell, the reverse process occurs: hydrogen and oxygen are combined to produce water, releasing electrical energy in the process. This relationship is significant because it demonstrates how fuel cells can be used as a clean and efficient means of energy conversion. By using hydrogen produced through electrolysis as a fuel, fuel cells can generate electricity with water vapor as the only byproduct, making them a promising technology for sustainable energy production.
Common types of Fuel Cells
Each type of fuel cell has its advantages and is suitable for different applications, contributing to the versatility of fuel cell technology.
- The alkaline (AFC) operates at room temperature and uses an alkaline electrolyte such as potassium hydroxide.
- The phosphoric acid (PAFC) uses phosphoric acid as the electrolyte and is often used in stationary power generation.
- The proton exchange membrane (PEMFC) uses a solid polymer membrane as the electrolyte and is commonly used in hydrogen cars due to its compact size and quick start-up time.
- Molten-carbonate (MCFCs) operate at high temperatures and use a molten carbonate electrolyte, making them suitable for large-scale power generation.
- Solid-oxide (SOFCs) operate at high temperatures and use a solid oxide electrolyte, offering high efficiency and fuel flexibility.
Alkali Fuel Cell
Alkaline FCs (AFCs) utilize compressed hydrogen and oxygen as fuel and potassium hydroxide (KOH) as the electrolyte. They operate at a relatively moderate temperature range of 150 ̊C to 200 ̊C, offering an output ranging from 300W to 5kW. These cells boast an efficiency of approximately 70%. Charge transport in AFCs primarily occurs through hydroxide ions (OH–).
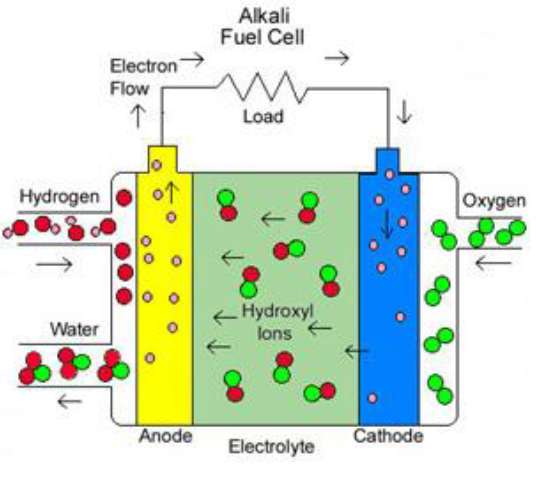
However, AFCs have specific requirements, including the need for pure hydrogen fuel and a platinum catalyst, which can be expensive. Additionally, the electrolyte being a liquid in AFCs can lead to corrosive leaks, necessitating careful maintenance and handling.
Phosphoric Acid Fuel Cell (PAFC)
Phosphoric Acid FCs (PAFCs) use phosphoric acid as the electrolyte and operate at temperatures between 150°C to 200°C. They are known for their efficiency, typically ranging from 40% to 80%. PAFCs have been tested in units as large as 11 MW. Charge transport in these cells primarily occurs through hydrogen ions (H+).
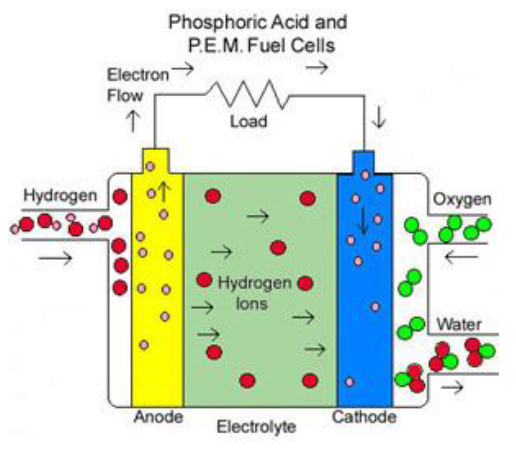
PAFCs are considered the most commercially successful cell system to date. However, their electrolyte is highly corrosive, requiring careful handling and maintenance. Additionally, the platinum catalyst used in PAFCs is very expensive, which adds to the cost of these systems.
Proton Exchange Membrane (PEM)
Proton Exchange Membrane (PEM) FCs use a thin, permeable polymer sheet as an electrolyte. The primary example of this is the Nafion® perfluorosulfonic acid membrane from DuPont. PEM cells typically operate at an efficiency of 40% to 50% and have a power output ranging from 50 to 250 kW. They operate at a relatively low temperature of 80°C, which is suitable for home or hydrogen car use.
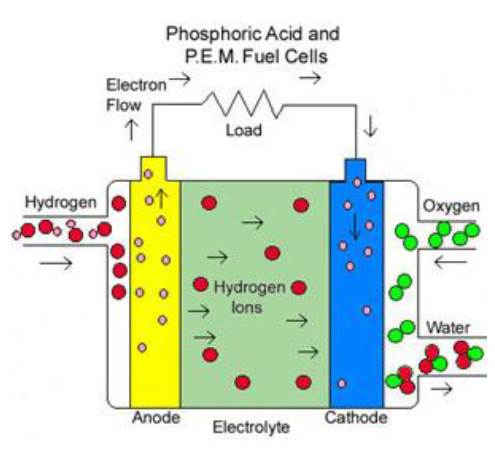
PEM fuel cells are considered the most suitable for transportation applications due to their compact size and quick start-up time. The electrolyte used in PEM cells will not leak or crack, enhancing their durability. However, the use of a platinum catalyst on both sides of the membrane adds to the cost of PEM fuel cells.
Molten Carbonate (MCFC)
Molten Carbonate FCs (MCFCs) use a unique electrolyte consisting of a mixture of alkali metal carbonates in a ceramic matrix, such as LiAlO2. These cells are known for their high efficiency, typically ranging from 60% to 80%. They operate at a high temperature of around 650°C, making them suitable for stationary energy generation applications. MCFCs utilize a cheap nickel electrode catalyst and can be constructed in units up to 2 MW, with designs for up to 100 MW.
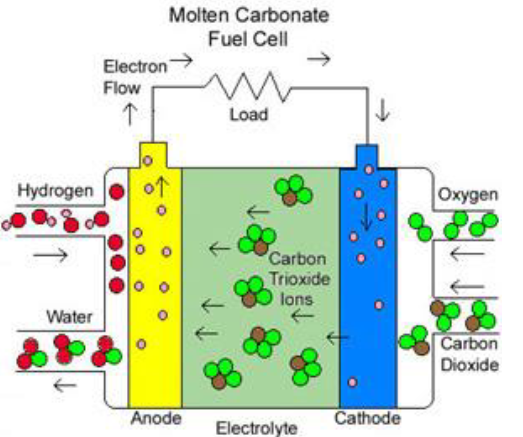
One of the key advantages of MCFCs is their ability to directly use hydrocarbon fuels. Charge transport in MCFCs occurs through carbonate ions (CO3-2). However, the operating temperature of MCFCs is too hot for many applications, and the carbonate ions are consumed in the reaction, requiring the injection of CO2 to compensate. Nevertheless, the high operating temperature allows for the use of non-precious metals as catalysts, reducing costs.
Solid Oxide (SOFC)
Solid Oxide FCs (SOFCs) employ a solid electrolyte made of yttria-stabilized ZrO2 (YSZ), a hard ceramic oxide. These cells operate at a high efficiency of approximately 60% and require a high operating temperature of around 1000°C. SOFCs can produce an output of up to 100 kW, making them suitable for various applications. Charge transport in SOFCs occurs through oxygen ions (O-2), and they use metal oxide electrocatalysts.

Additionally, SOFCs can directly use hydrocarbon fuels. They are being developed for both stationary and portable applications. The high operating temperature of SOFCs allows the catalyst to extract hydrogen from the fuel at the electrode, increasing efficiency. However, this high temperature limits their use in certain applications. SOFC units are typically large, and while the solid electrolyte won’t leak, it can be susceptible to cracking.
What is the problem with Solid Oxide fuel cells?
These cells are in the early stages of development, facing challenges particularly in SOFC systems due to their high operating temperatures. One significant challenge is the potential buildup of carbon dust on the anode, which can hinder the internal reforming process.
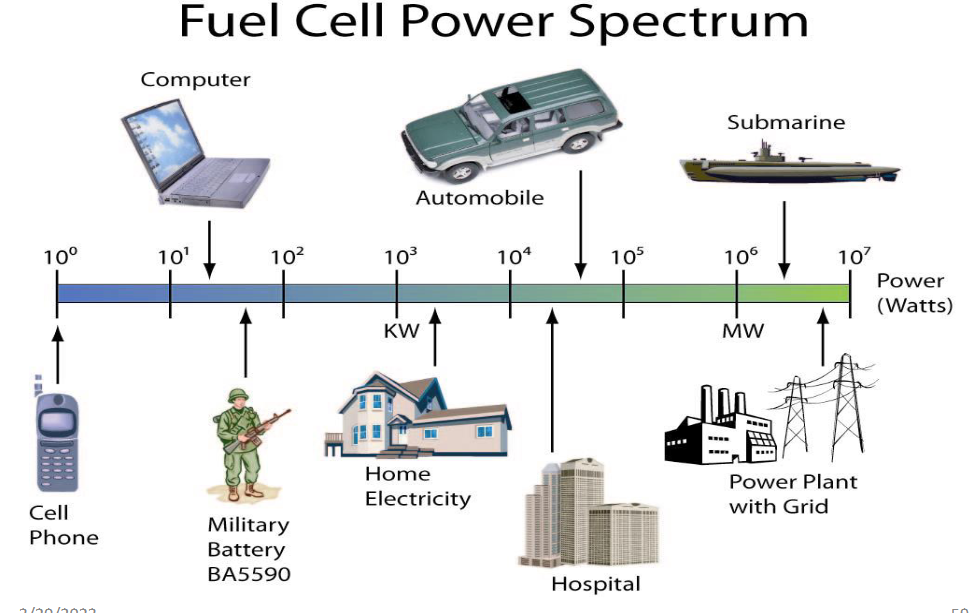
Examples of Fuel Cell Application
Fuel cells find diverse applications across the value chain, including material handling in distribution centers, stationary power generation in various settings, and both micro- and macro-mobility solutions. Digging a little deeper into the mobility solutions; fuel cells can power a wide range of vehicles. For example personal cars, large trucks or buses, marine vessels, and specialty vehicles like lift trucks and ground support equipment. They can also serve as auxiliary power units for conventional transportation technologies.
Space Systems & Fuel Cells
Fuel cells have been crucial components in space systems, with notable examples including the 1.5 kW Apollo fuel cell, of which two were used in the Apollo spacecraft. These cells were essential for providing electrical power and water during the Apollo missions.
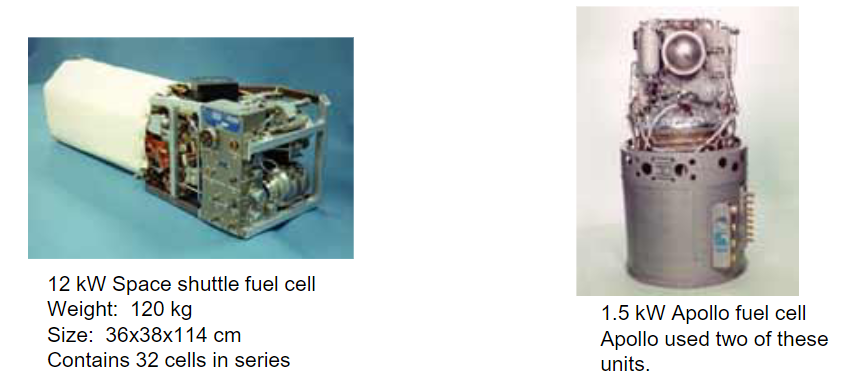
Another significant example is the 12 kW cells used in the Space Shuttle program, which provided power for the shuttle’s electrical systems, including lighting, communication, and life support systems. These fuel cells weigh around 120 kg and measures 36x38x114 cm, and contain 32 cells connected in series to generate the required power for space missions.
Fuel Cells in Use: Portable Systems
Fuel cells are increasingly finding applications in portable systems, offering longer run times compared to traditional batteries. For example, a laptop powered by a cell can operate for up to 20 hours on a single charge of fuel. This extended runtime is particularly beneficial for users who require their devices to remain operational for extended periods without access to traditional power sources.

Stationary Power Supply Units.
Stationary systems have become increasingly popular worldwide, with over 2500 installations in various settings such as hospitals, nursing homes, hotels, office buildings, schools, utility power plants, and even an airport terminal. These systems serve as either primary power sources or backups, offering reliable electricity generation. In large-scale building systems, fuel cells have demonstrated the ability to reduce energy service costs by a significant margin—between 20% to 40%—when compared to conventional energy sources. These cost savings, along with their reliability, have made fuel cells an attractive option for many commercial and institutional applications.

However, the adoption of residential cell power units has been slower. While there are few units available on the market, many designs are currently undergoing testing and are expected to be available in the coming years. One of the major challenges in producing residential cells is ensuring their safety for installation in homes and making them easy to maintain for the average homeowner. These units are typically the size of a large deep freezer or furnace, such as the Plug Power 7000 unit, and cost between $5000 to $10,000.

For residential systems to be economically viable for power companies, they would need to charge homeowners at least 40 cents per kilowatt-hour (¢/kWh). This pricing model would likely relegate residential fuel cells to backup power supply status for the foreseeable future, as grid electricity remains more cost-effective for primary power needs. However, as technology advances and economies of scale come into play, residential fuel cells could become more prevalent, offering homeowners a reliable and potentially cost-effective alternative to traditional grid electricity.
Conclusion
In conclusion, fuel cells represent a promising and innovative technology with the potential to revolutionize energy production and transportation. By converting chemical energy directly into electricity, water, and heat through electrochemical reactions, fuel cells offer a clean, efficient, and sustainable alternative to traditional combustion-based power sources.
Despite facing challenges such as high operating temperatures and cost, ongoing research and development efforts continue to improve fuel cell technology, making it increasingly viable for a wide range of applications. As we strive towards a greener future, fuel cells stand out as a key player in the transition to a more sustainable energy landscape.